Izotermik mikrokalorimetriya - Isothermal microcalorimetry
 | |
| Qisqartma | IMC |
|---|---|
| Tasnifi | Termal tahlil |
| Boshqa usullar | |
| Bog'liq | Izotermik titrlash kalorimetri Differentsial skanerlash kalorimetri |
Izotermik mikrokalorimetriya (IMC) bu kimyoviy, fizik va biologik jarayonlarni real vaqt rejimida kuzatish va dinamik tahlil qilish uchun laboratoriya usuli. Bir necha soat yoki kun davomida IMC kichik hajmdagi namunalar uchun bunday jarayonlarning boshlanishini, tezligini, hajmini va energetikasini aniqlaydi. ampulalar (masalan, 3-20 ml) doimiy belgilangan haroratda (taxminan 15 ° C-150 ° C).
IMC bu dinamik tahlilni o'tgan vaqtni va ampuladan namunaga yoki undan issiqlik oqimining sof tezligini (mJJ / sek = mW) va iste'mol qilingan yoki ishlab chiqarilgan issiqlikning (J) yig'indisi miqdorini o'lchash va qayd etish orqali amalga oshiradi.
IMC bir-biri bilan chambarchas bog'liq to'rt sababga ko'ra kuchli va ko'p qirrali analitik vositadir:
- Barcha kimyoviy va fizik jarayonlar ekzotermik yoki endotermikdir - issiqlik hosil qiladi yoki iste'mol qiladi.
- The issiqlik oqimining tezligi sodir bo'layotgan jarayon tezligiga mutanosibdir.
- IMC bir necha gramm materialdagi sekin jarayonlarni (yiliga bir necha foizga boradigan) yoki minus issiqlik hosil qiladigan jarayonlarni (masalan, bir necha ming tirik hujayralar metabolizmi) aniqlash va kuzatib borish uchun etarlicha sezgir.
- IMC asboblari odatda ulkan dinamik diapazonga ega - issiqlik oqimi taxminan CA ga teng. 1 mVt va taxminan yuqori. Xuddi shu asbob bilan 50 000 mVtni o'lchash mumkin.
Jarayonlarning stavkalarini o'rganishning IMC usuli shu tarzda keng qo'llaniladi, real vaqtda doimiy ma'lumot beradi va sezgir. O'lchovni bajarish oson, qarovsiz amalga oshiriladi va aralashmaydi (masalan, lyuminestsent yoki radioaktiv markerlar kerak emas).
Shu bilan birga, IMC-dan foydalanishda ikkita asosiy ogohlantirish mavjud:
- O'tkazib yuborilgan ma'lumotlar: Agar tashqi tomondan tayyorlangan ampuladan foydalanilsa, u taxminan O'lchash modulida belgilangan haroratni sezilarli darajada buzmasdan asbobga ampulani asta-sekin kiritish uchun 40 daqiqa. Shunday qilib, shu vaqt ichida sodir bo'lgan har qanday jarayonlar kuzatilmaydi.
- Tashqi ma'lumotlar: IMC ampulada sodir bo'lgan barcha jarayonlar tomonidan ishlab chiqarilgan yoki iste'mol qilingan umumiy issiqlik oqimini qayd etadi. Shu sababli, o'lchangan issiqlik oqimini qanday jarayon yoki jarayonlar hosil qilayotganiga amin bo'lish uchun eksperimental loyihalashda ham, tegishli kimyoviy, fizik va biologik tahlillardan dastlabki foydalanishda ham juda ehtiyot bo'lish kerak.
Umuman olganda, IMC-ning mumkin bo'lgan dasturlari faqat analitik vosita sifatida foydalanishni tanlagan kishining tasavvurlari va usulning jismoniy cheklovlari bilan cheklanadi. Yuqorida tavsiflangan ikkita umumiy cheklovlardan (asosiy ogohlantirishlar) tashqari, ushbu cheklovlarga namuna va ampulaning kattaligi va o'lchovlarni o'tkazish mumkin bo'lgan harorat kiradi. IMC odatda soatlab yoki bir necha kun davomida sodir bo'lgan jarayonlarni baholash uchun eng mos keladi. IMC juda keng ko'lamda ishlatilgan va ko'plab maqolalar ushbu maqolada muhokama qilingan bo'lib, nashr etilgan adabiyotlarga havolalar bilan tasdiqlangan. Ilovalar polimerlarning sekin oksidlanish darajasidagi parchalanishini va xavfli sanoat kimyoviy moddalarining beqarorligini o'lchashdan siydikdagi bakteriyalarni aniqlash va parazit qurtlarga dorilar ta'sirini baholashgacha muhokama qilindi. Ushbu maqoladagi asosiy e'tibor so'nggi turga tegishli bo'lgan biologiya va tibbiyotga tegishli.
Umumiy nuqtai
Ta'rifi, maqsadi va ko'lami
Kalorimetriya o'lchov haqidagi fan kimyoviy reaktsiyalarning issiqligi yoki jismoniy o'zgarishlar. Kalorimetriya a bilan amalga oshiriladi kalorimetr.
Izotermik mikrokalorimetriya (IMC) - bu real vaqt rejimida, issiqlik oqimining tezligini (mJ / sek = mW) va doimiy ravishda haroratda iste'mol qilingan yoki ishlab chiqarilgan issiqlikning yig'indisi (J) ni doimiy ravishda o'lchash uchun laboratoriya usuli, bu IMCga joylashtirilgan namuna. asbob. Bunday issiqlik namunadagi kimyoviy yoki fizikaviy o'zgarishlarga bog'liq. Issiqlik oqimi ma'lum bir vaqtda sodir bo'lgan o'zgarishlarning umumiy tezligiga mutanosibdir. Belgilangan vaqt oralig'ida hosil bo'lgan umumiy issiqlik sodir bo'lgan agregat o'zgarishlarining yig'ma miqdoriga mutanosibdir.
Shunday qilib, IMC biologik jarayonlarni o'z ichiga olgan keng ko'lamli tezlik jarayonlarining stavkalari va energetikasini dinamik, miqdoriy baholash vositasidir. Tezlik jarayoni bu erda fizik va / yoki kimyoviy o'zgarish sifatida aniqlanadi, uning vaqt o'tishi bilan rivojlanishi empirik yoki matematik model bilan tavsiflanishi mumkin (Bibliografiya: Glasstone va boshq. 1941 va Jonson va boshqalar. 1974 va Tezlik tenglamasi ).
IMC-ning eng oddiy ishlatilishi - bu namunada bir yoki bir nechta tezlik jarayonlari sodir bo'lishini aniqlash, chunki issiqlik ishlatilgan asbobni aniqlash chegarasidan yuqori tezlikda ishlab chiqariladi yoki iste'mol qilinadi. Bu, masalan, qattiq yoki suyuq materialning inert emasligi, aksincha ma'lum bir haroratda o'zgarib turishini ko'rsatadigan umumiy ko'rsatkich sifatida foydali bo'lishi mumkin. O'sish muhitini o'z ichiga olgan biologik namunalarda, vaqt o'tishi bilan aniqlanadigan va ko'tarilgan issiqlik oqimi signalining ko'rinishi ba'zi bir takrorlanadigan hujayralar mavjudligining oddiy umumiy ko'rsatkichidir.

Shakl.1
Biroq, aksariyat ilovalar uchun qandaydir usul yoki jarayonlar issiqlik oqimini kuzatish orqali qanday o'lchov qilinishini bilish muhimdir. Umuman olganda, bu avval IMC ampulasiga joylashtirilgan buyumlar haqida vaqt o'tishi bilan issiqlik oqimini baholash uchun IMC asbobiga joylashtirilgunga qadar fizikaviy, kimyoviy va biologik ma'lumotlarga ega bo'lishni talab qiladi. Bundan tashqari, bir yoki bir necha vaqt davomida issiqlik oqimining IMC o'lchovlari o'tkazilgandan keyin ampula tarkibini tahlil qilish kerak. Shuningdek, ampula tarkibidagi mantiqiy o'zgarishlardan issiqlik oqimining o'ziga xos manbasini yoki manbalarini aniqlash uchun foydalanish mumkin. Tezlik jarayoni va issiqlik oqimi munosabatlari o'rnatilganda, to'g'ridan-to'g'ri IMC ma'lumotlariga ishonish mumkin.
IMC amalda nimani o'lchashi mumkin, qisman namuna o'lchamlariga bog'liq va ular asboblar dizayni bilan cheklanishi shart. Ushbu tijorat vositasi odatda belgilangan diametr va balandlikgacha namunalarni qabul qiladi. O'lchamlari ca gacha bo'lgan namunalarni qabul qiladigan asboblar. Diametri x yoki taxminan 1 yoki 2 sm. Balandligi 5 sm. Berilgan asbobda berilgan turdagi kattaroq namunalar odatda ko'proq issiqlik oqimi signallarini hosil qiladi va bu aniqlanish va aniqlikni oshirishi mumkin.
Tez-tez namunalar 3 dan 20 ml gacha bo'lgan silindrsimon ampulalarni oddiy (1-rasm) o'z ichiga oladi, ularning tezligi jarayonlari qiziqish uyg'otadi, masalan. qattiq moddalar, suyuqliklar, o'stirilgan hujayralar yoki issiqlik ishlab chiqarish yoki iste'mol qilishga olib kelishi kutilayotgan bu yoki boshqa narsalarning har qanday birikmasi. Ko'pgina foydali IMC o'lchovlari oddiy muhrlangan ampulalar yordamida amalga oshirilishi mumkin va shisha ampulalari keng tarqalgan, chunki shisha issiqlik hosil qiluvchi kimyoviy yoki fizik o'zgarishlarga moyil emas. Biroq, ba'zida metall yoki polimerik ampulalar qo'llaniladi. Shuningdek, gazlar yoki suyuqliklarni quyish yoki boshqarish orqali boshqariladigan va / yoki namunani mexanik aralashtirishni ta'minlaydigan asbob / ampula tizimlari mavjud.
Tijorat IMC asboblari issiqlik oqimini o'lchash uchun haroratni CA dan farq qiladi. 15 ° C - 150 ° C. Muayyan asbobning diapazoni biroz boshqacha bo'lishi mumkin.
IMC juda sezgir - masalan. Bir necha gramm og'irlikdagi namunalardagi sekin kimyoviy reaktsiyalar natijasida paydo bo'ladigan issiqlik, reaktiv moddalarni iste'mol qilish tezligi yiliga bir necha foizni tashkil etganda, bir necha kun ichida aniqlanishi va miqdori aniqlanishi mumkin. Masalan, polimer implant materiallarining asta-sekin oksidlanishini va qattiq farmatsevtika dori formulalarini saqlash muddatini o'rganish (Ilovalar: Qattiq materiallar ).
Shuningdek, masalan, metabolik issiqlik ishlab chiqarish darajasi. IMC ampulasida madaniyatdagi bir necha ming tirik hujayralarni, mikroorganizmlarni yoki protozoyalarni o'lchash mumkin. Bunday metabolik issiqlik miqdori mavjud hujayralar yoki organizmlar soni bilan (tajriba orqali) o'zaro bog'liq bo'lishi mumkin. Shunday qilib, IMC ma'lumotlari real vaqt rejimida mavjud bo'lgan hujayralar yoki organizmlar sonini va ushbu sonning o'sish yoki pasayishning aniq tezligini kuzatish uchun ishlatilishi mumkin (Ilovalar: Biologiya va tibbiyot ).
IMCning ba'zi biologik bo'lmagan dasturlari muhokama qilinsa ham (Ilovalar: Qattiq materiallar ) ushbu maqoladagi hozirgi e'tibor IMC-ni biologik jarayonlar bilan bog'liq holda ishlatishga qaratilgan (Ilovalar: Biologiya va tibbiyot ).
Olingan ma'lumotlar

Shakl.2
IMC ma'lumotlarining keng tarqalgan turlarining grafik displeyi 2-rasmda keltirilgan. Yuqorida muhrlangan ampuladagi namunadan vaqtga nisbatan yozilgan issiqlik oqimi (m Vt = m J / s) ko'rsatilgan. ekzotermik tezlik jarayoni boshlanib, tezlashadi, eng yuqori issiqlik oqimiga etadi va keyin susayadi. Bunday ma'lumotlar to'g'ridan-to'g'ri foydalidir (masalan, aniqlangan sharoitda jarayonni aniqlash va uning davomiyligi), ammo jarayon parametrlarini aniqlash uchun ma'lumotlar matematik jihatdan osonlikcha baholanadi. Masalan, 2-rasm, shuningdek, issiqlik oqimi ma'lumotlarining integratsiyasini ko'rsatadi va to'plangan issiqlik (J) ni vaqtga nisbatan beradi. Ko'rsatilganidek, jarayonning maksimal o'sishi (issiqlik hosil qilish) tezligi va jarayon maksimal issiqlikka yetguncha kechikish fazasining davomiyligi kabi parametrlarni integral ma'lumotlar asosida hisoblash mumkin.[1] Kompyuter fayllari sifatida saqlanadigan issiqlik oqimi tezligi ma'lumotlari yordamida hisob-kitoblar osonlikcha avtomatlashtiriladi. O'sish parametrlarini aniqlash uchun IMC ma'lumotlarini shu tarzda tahlil qilish hayot fanlari uchun muhim dasturlarga ega (Ilovalar: Biologiya va tibbiyot ). Shuningdek, bir qator haroratlarda olingan issiqlik oqimining tezligi baholanadigan jarayonning faollashuv energiyasini olish uchun ishlatilishi mumkin (Hardison va boshq. 2003).[2]
Rivojlanish tarixi
Lavoazye va Laplas taxminan izotermik kalorimetrni yaratgan va ishlatgan. 1780 (Bibliografiya: Lavoisier A & Laplace PS 1780 ). Ularning asboblari cheklangan makonda nisbatan doimiy haroratni hosil qilish uchun muzdan foydalangan. Ular issiqlik hosil qiluvchi namunani muzga (masalan, tirik hayvonga) qo'yganlarida, eritayotgan muzdan hosil bo'lgan suyuq suv massasi namunadagi issiqlik bilan to'g'ridan-to'g'ri proportsionalligini angladilar.
Ko'plab zamonaviy IMC asboblari dizaynlari Shvetsiyada 1960 yillarning oxiri va 1970 yillarning boshlarida amalga oshirilgan (Wadsö 1968,[3] Suurkuusk va Wadsö 1974 yil[4]). Ushbu ish qattiq jismli elektron qurilmalarning parallel rivojlanishidan, xususan kichiklarning tijorat mavjudligidan foydalangan termoelektrik ta'sir (Peltier-Seebeck) issiqlik oqimini kuchlanishga aylantirish uchun moslamalar va aksincha.
1980-yillarda ko'p kanalli dizaynlar paydo bo'ldi (Suurkuusk 1982),[5] bir nechta namunalarni parallel ravishda baholashga imkon beradi. Bu IMC-ning kuchini va foydaliligini sezilarli darajada oshirdi va uslubni aniq sozlash uchun harakatlarga olib keldi (Thoren va boshq. 1989).[6] 1990-yillarda amalga oshirilgan loyihalashtirish va ishlab chiqish ishlarining aksariyati Shvedda Vadso va Suurkuusk va ularning hamkasblari tomonidan amalga oshirildi. Ushbu ish shaxsiy kompyuter texnologiyasining parallel rivojlanishidan foydalanib, issiqlik oqimini vaqt ma'lumotlariga nisbatan osongina saqlash, qayta ishlash va izohlash qobiliyatini sezilarli darajada oshirdi.
1990-yillardan boshlab asbobsozlik ishlari qattiq jismlar elektronikasi va shaxsiy kompyuter texnologiyalarining doimiy rivojlanishidan yanada ko'proq foyda oldi. Bu IMC sezgirligi va barqarorligini oshiruvchi vositalarni, parallel kanallar sonini va IMC ma'lumotlarini qulay tarzda yozib olish, saqlash va tezda qayta ishlash qobiliyatini yaratdi. Kengroq foydalanish bilan bog'liq holda, IMC asboblarining ishlashini tavsiflash uchun standartlarni yaratishga (masalan, aniqlik, aniqlik, sezgirlik) va kalibrlash usullariga katta e'tibor berildi (Wadsö va Goldberg 2001).[7]
Asboblar va o'lchov tamoyillari
Asboblarning konfiguratsiyasi
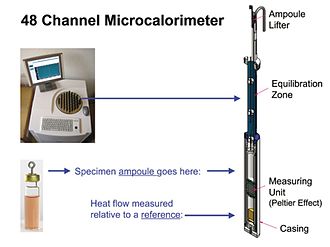
Shakl.3
Zamonaviy IMC asboblari aslida yarim adiabatikdir, ya'ni. namuna va uning atrofi o'rtasida issiqlik uzatish nolga teng emas (adiabatik), chunki issiqlik oqimining IMC o'lchami kichik harorat differentsial-ca mavjudligiga bog'liq. 0.001 ° S.[7] Biroq, differentsial juda past bo'lganligi sababli, IMC o'lchovlari asosan izotermikdir. 3. rasmda 48 ta issiqlik oqimini o'lchash modullarini o'z ichiga olgan IMC asbobining umumiy ko'rinishi ko'rsatilgan. Bitta modul ko'rsatilgan. Modulning o'lchov birligi odatda Peltier-Seebeck qurilmasidir. Qurilma issiqlik chiqaradigan yoki iste'mol qiladigan namuna va issiqlik batareyasining haroratida bo'lgan termal faol bo'lmagan ma'lumotnoma orasidagi harorat farqiga mutanosib kuchlanish hosil qiladi. Haroratning farqi o'z navbatida namunaning issiqlik ishlab chiqarish yoki iste'mol qilish tezligiga mutanosibdir (quyida kalibrlashga qarang). Asbobning barcha modullari bir xil issiqlik batareyasi va termostatni ishlatadi va shu bilan ularning barchasi bir xil belgilangan haroratda ma'lumot ishlab chiqaradi. Biroq, odatda har bir ampulada o'lchovlarni mustaqil ravishda boshlash va to'xtatish mumkin. Shakl 3da ko'rsatilgandek yuqori darajada parallel (masalan, 48 kanalli) asbobda, bu qulay bo'lgan paytda bir nechta turli xil tajribalarni amalga oshirish (boshlash va to'xtatish) imkonini beradi.
Shu bilan bir qatorda, IMC asboblari ikkita ampula orasidagi issiqlik oqimi farqiga mutanosib signal beradigan dupleks modullar bilan jihozlanishi mumkin. Ikkita dupleks ampuladan biri ko'pincha bo'sh yoki nazoratdir, ya'ni. foiz stavkasi jarayonini ishlab chiqaradigan materialni o'z ichiga olmaydigan, ammo uning mazmuni aks holda ampuladagi namunaga o'xshash bo'lgan namunadir. Bu kichik issiqlik hosil qiluvchi reaktsiyalarni yo'q qilish uchun vositani beradi, masalan, qiziqish uyg'otmaydi, masalan, hujayra madaniyati muhitida bir necha kun davomida o'lchov haroratida asta-sekin kimyoviy o'zgarishlar. Ko'pgina foydali IMC o'lchovlari oddiy muhrlangan ampulalar yordamida amalga oshirilishi mumkin. Biroq, yuqorida aytib o'tilganidek, gazlar yoki suyuqliklarning namunalarga va / yoki ulardan oqishini ta'minlaydigan yoki hatto boshqaradigan va / yoki namunani mexanik aralashtirishni ta'minlaydigan asbob / ampula tizimlari mavjud.
Malumot qo'shimchalari
Issiqlik oqimi, odatda, 3-rasmda ko'rsatilgandek mos yozuvlar qo'shimchasiga nisbatan o'lchanadi. Bu odatda metalldir kupon bu asbobning ishlash doirasidagi har qanday haroratda kimyoviy va fizik jihatdan barqaror va shuning uchun o'zi issiqlik hosil qilmaydi yoki iste'mol qilmaydi. Eng yaxshi ishlash uchun mos yozuvlar namunaga yaqin issiqlik quvvatiga ega bo'lishi kerak (masalan, IMC ampulasi plyus tarkibi).
Ish tartibi
Issiqlik o'tkazuvchanligi (hc) rejimi
Savdo IMC asboblari ko'pincha issiqlik o'tkazuvchanligi (hc) kalorimetri sifatida ishlaydi, unda namuna tomonidan ishlab chiqarilgan issiqlik (ya'ni ampuladagi material) issiqlik qabul qiluvchiga, odatda termostat tarkibidagi alyuminiy blokga oqib chiqadi (masalan, doimiy haroratli hammom). Yuqorida aytib o'tganimizdek, hc rejimida ishlaydigan IMC vositasi aniq izotermik emas, chunki belgilangan harorat va namuna harorati o'rtasidagi kichik farqlar albatta mavjud bo'lib, o'lchanadigan issiqlik oqimi mavjud. Shu bilan birga, namunadagi haroratning kichik o'zgarishlari issiqlik batareyasining haroratiga sezilarli ta'sir ko'rsatmaydi, chunki issiqlik qabul qilgichning issiqlik quvvati namunadan ancha yuqori - odatda taxminan. 100 ×.
Namuna va issiqlik qabul qiluvchisi o'rtasida issiqlik almashinuvi a orqali amalga oshiriladi Peltier-Seebeck ishlab chiqarilgan yoki iste'mol qilinadigan issiqlikni dinamik ravishda o'lchashga imkon beruvchi qurilma. Tadqiqot sifatli asboblarda termostat / sovutgichning harorati odatda <± 0,1 K ga to'g'ri keladi va taxminan taxminan saqlanadi. <± 100 mK / 24 soat. Vaqt o'tishi bilan issiqlik qabul qiluvchisi harorati saqlanib turadigan aniqlik vaqt o'tishi bilan issiqlik oqimi o'lchovlarining aniqligini belgilovchi asosiy omil hisoblanadi. HC rejimining afzalligi bu katta dinamik diapazon. Taxminan issiqlik oqimlari. 50,000 m Vt ni aniqlik bilan o'lchash mumkin. ± 0,2 mVt. Shunday qilib, ca ning issiqlik oqimini o'lchash. > Dastlabki ko'rsatkichdan 0,2 mVt yuqori issiqlik oqimini aniqlashni tashkil etadi, ammo 10 × aniqlik chegarasini konservativ aniqlash tez-tez ishlatiladi.
Quvvatni kompensatsiya qilish (kompyuter) rejimi
Ba'zi IMC asboblari quvvat kompensatsiyasi (kompyuter) kalorimetri sifatida ishlaydi (yoki ishlatilishi mumkin). Bunda namunani belgilangan haroratda ushlab turish uchun ishlab chiqarilgan issiqlik Peltier-Seebeck moslamasi yordamida qoplanadi. Iste'mol qilinadigan issiqlik elektr toki bilan yoki qurilmaning qutblanishini qaytarish bilan qoplanadi (van Herwaarden, 2000).[8] Agar berilgan asbob hc emas, balki kompyuter rejimida ishlasa, issiqlik oqimini o'lchash aniqligi bir xil bo'lib qoladi (masalan, taxminan ± 0,2 mVt). Kompensatsiya rejimining afzalligi kichikroq vaqt doimiyligidir - ya'ni ma'lum bir issiqlik oqimi pulsini aniqlash uchun zarur bo'lgan vaqt o'tkazuvchanlik rejimiga qaraganda taxminan 10X qisqa. Kamchilik - bu HC rejimiga nisbatan 10 baravar kichikroq dinamik diapazon.
Kalibrlash
Hc yoki kompyuter rejimida ishlash uchun tijorat asboblarida muntazam kalibrlash odatda o'rnatilgan elektr isitgichlar bilan amalga oshiriladi. Elektr isitgichlarining ishlashi, o'z navbatida, ma'lum issiqlik quvvati namunalari yordamida yoki issiqlik reaktsiyasi hosil bo'lgan kimyoviy reaktsiyalarni ishlab chiqarishi mumkin, ularning massa birligi uchun issiqlik hosil bo'lishi termodinamikadan ma'lum (Wadsö va Goldberg 2001).[7] Hc yoki pc rejimida olingan signal kompyuterning yozib olinadigan voltajidir, u m W W-diapazonidagi issiqlik oqimi va vaqtini belgilash uchun sozlangan. Xususan, agar namunada sezilarli issiqlik gradyanlari mavjud bo'lmasa, u holda P = eC [U + t (dU / dt)], bu erda P - issiqlik oqimi (ya'ni m V Vt), phC - kalibrlash doimiysi, U termopile bo'yicha o'lchangan potentsial farqi va t vaqt doimiysi. Barqaror holat sharoitida - masalan, doimiy elektr kalibrlash tokining chiqishi paytida bu P = e ga soddalashtiradiC U. (Wadsö va Goldberg 2001).[7]
Ampulalar
Ko'pgina juda foydali IMC o'lchovlari muhrlangan ampulalarda o'tkazilishi mumkin (1-rasm), bu oddiylik, ifloslanishdan himoya qilish va (agar kerak bo'lsa) ampulalar bilan muomala qiladigan yoki ta'sir qiladigan odamlar uchun bio-xavfsizlikning katta chegaralarini beradi. Yopiq ampulada qattiq moddalar, suyuqliklar, gazlar yoki biologik kelib chiqadigan narsalarning istalgan birikmasi bo'lishi mumkin. Ampulaning bosh bo'shlig'idagi dastlabki gaz tarkibi ampulani kerakli gaz muhitida muhrlash orqali boshqarilishi mumkin.
Shu bilan birga, o'lchov va / yoki mexanik aralashtirish paytida ampuladan gaz yoki suyuqlikni boshqariladigan oqimiga ruxsat beruvchi IMC asboblari / ampulalarining konstruktsiyalari ham mavjud. Shuningdek, tegishli aksessuarlar bilan ba'zi IMC asboblari ITC (izotermik titrlash kalorimetri) asboblari sifatida ishlatilishi mumkin. ITC mavzusi boshqa joylarda yoritilgan (qarang Izotermik titrlash kalorimetri ). Bundan tashqari, ba'zi bir IMC asboblari vaqt o'tishi bilan harorat asta-sekin o'zgartirilganda (skanerlashda) issiqlik oqimini yozib olishlari mumkin. Skanerlash tezligi sekin bo'lishi kerak. IMC shkalasi namunalarini (masalan, bir necha gramm) issiqlik qabul qiluvchisi haroratiga (<0,1 ° C) etarlicha yaqin tutish uchun ± 2 K ° / soat. Haroratni tez skanerlash viloyatidir differentsial skanerlash kalorimetri Odatda juda kichik namunalarni ishlatadigan (DSC) asboblar. Ba'zi DSC asboblarini IMC rejimida ishlatish mumkin, ammo skanerlash uchun zarur bo'lgan kichik ampulaning (va shuning uchun namunaning) o'lchamlari IMC rejimida ishlatiladigan DSC asboblarining foydali va sezgirligini cheklaydi.
Asosiy metodologiya
Haroratni sozlash
Issiqlik oqimi tezligini (mJ / sek = mVt) o'lchash birinchi navbatda IMC asbob termostatini tanlangan haroratda sozlash va asbobning issiqlik qabul qiluvchisini shu haroratda barqarorlashtirishga imkon berish orqali amalga oshiriladi. Agar bir haroratda ishlaydigan IMC vositasi yangi haroratga o'rnatilsa, yangi harorat rejimida qayta stabillash bir necha soat, hatto bir kun davom etishi mumkin. Yuqorida aytib o'tilganidek, aniq barqaror haroratga erishish va uni ushlab turish uzoq vaqt davomida (masalan, kunlar) mW oralig'ida aniq issiqlik oqimi o'lchovlariga erishish uchun muhimdir.
Namuna bilan tanishtirish
Haroratni barqarorlashtirgandan so'ng, agar tashqi tomondan tayyorlangan ampuladan (yoki ampulaning ba'zi bir qattiq namunalaridan) foydalanilsa, u asta-sekin asbobning o'lchov moduliga kiritiladi (masalan, tushiriladi), odatda bosqichli operatsiya. Maqsad ampula / namuna o'lchov holatiga kelguniga qadar uning harorati o'lchov haroratiga (0,001 ° C atrofida) yaqin bo'lishini ta'minlashdir. Shunday qilib, o'lchangan har qanday issiqlik oqimi namunani belgilangan haroratga etkazishning davom etadigan jarayoni tufayli emas, balki namunalar tezligi jarayonlari bilan bog'liq. 3-20 ml IMC ampulasida namunani o'lchov holatiga kiritish vaqti taxminan. Ko'pgina asboblarda 40 daqiqa. Bu shuni anglatadiki, ushbu kirish davrida namuna ichida sodir bo'lgan har qanday jarayonlardan issiqlik oqimi qayd etilmaydi.
Agar joyida ampuladan foydalanilsa va ba'zi bir vositalar yoki namunalar AOK qilingan bo'lsa, bu ham beqarorlik davrini keltirib chiqaradi, ammo u buyurtma bo'yicha. 1 daqiqa. 5-rasmda to'g'ridan-to'g'ri ampula kiritilsa, asbobni barqarorlashtirish uchun zarur bo'lgan uzoq muddat va in'ektsiya tufayli qisqa muddatli beqarorlik misollari keltirilgan.
Ma'lumotlarni yozib olish
Kirish jarayonidan so'ng, namunadagi issiqlik oqimi doimiy ravishda aniqlanishi mumkin, chunki bu qiziqish uyg'otadi. Tadqiqot darajasidagi asboblarning o'ta barqarorligi (<± 100 mkK / 24 soat) bir necha kun davomida aniq o'lchovlarni (va ko'pincha) amalga oshirish mumkinligini anglatadi. Issiqlik oqimi signali real vaqt rejimida o'qilishi mumkin bo'lganligi sababli, u qiziqishning issiqlik oqimi hali ham sodir bo'ladimi yoki yo'qligini hal qilish uchun vosita bo'lib xizmat qiladi. Shuningdek, zamonaviy asboblar issiqlik oqimi va vaqt ma'lumotlarini kompyuter fayllari sifatida saqlaydi, shuning uchun ham real vaqtda, ham retrospektiv grafik displey va ma'lumotlarni matematik tahlil qilish mumkin.
Foydalanish imkoniyati
Quyida aytib o'tilganidek, IMC tezlikni jarayonlarini tahlil qilish usuli sifatida juda ko'p afzalliklarga ega, ammo ba'zi bir ogohlantirishlarga ham e'tibor berish kerak.
Afzalliklari
Keng tarqalgan
Har qanday tarif jarayonini o'rganish mumkin - agar mos namunalar IMC asbob moduli geometriyasiga to'g'ri kelsa va IMC metodologiyasiga mos keladigan tezliklarda davom etsa (yuqoriga qarang). Ko'rsatilganidek Ilovalar, IMC in vitro stavka jarayonlarining juda keng doirasini aniqlash uchun foydalanilmoqda, masalan. polimerlarning qattiq holatdagi barqarorligidan (Hardison va boshq. 2003)[2] parazit qurtlarga qarshi dori birikmalarining samaradorligiga (Maneck va boshq. 2011).[9] IMC shuningdek xarakterlanmagan, murakkab yoki ko'p sonli o'zaro ta'sirlarning umumiy tezligini aniqlay oladi (Lyuis va Daniels).[10] Bu, ayniqsa, qiyosiy skrining uchun foydalidir - masalan. moddiy tarkib va / yoki ishlab chiqarish jarayonlarining turli xil birikmalarining umumiy fizik-kimyoviy barqarorlikka ta'siri.
Haqiqiy vaqtda va doimiy
IMC issiqlik oqimi ma'lumotlari voltaj o'zgarishi va vaqt o'zgarishi bilan olinadi, kompyuter fayllari sifatida saqlanadi va real vaqtda ko'rsatilishi mumkin, chunki stavka jarayoni sodir bo'ladi. Issiqlik oqimi bilan bog'liq kuchlanish vaqt o'tishi bilan uzluksiz, ammo zamonaviy asboblarda u odatda raqamli ravishda namuna olinadi. Raqamli namuna olish chastotasini kerak bo'lganda boshqarish mumkin, ya'ni. ma'lumotlar fayli hajmini cheklash uchun vaqtni yaxshiroq aniqlash yoki sekin o'zgarishlarni sekinroq tanlash uchun tez issiqlik oqimining tez-tez o'zgarishi.
Nozik va tezkor
IMC uzoq vaqt (oylar) davomida reaktiv moddalarning atigi bir necha foizini iste'mol qiladigan reaktsiyalarni qisqa vaqt ichida (soat, kun) aniqlash va miqdorini aniqlash uchun etarlicha sezgir. Shunday qilib, IMC odatdagi (masalan, kimyoviy) tahlillar uchun etarli reaksiya mahsuloti to'planguniga qadar uzoq kutishdan qochadi. Bu fizik va biologik namunalarga tegishli (qarang Ilovalar ).
To'g'ridan-to'g'ri
Namuna o'zgaruvchilarining har bir kombinatsiyasida va qiziqishning belgilangan haroratida IMC issiqlik oqimlari kinetikasi va tezlik jarayonlarining to'plangan issiqligini to'g'ridan-to'g'ri aniqlashni ta'minlaydi. Bu IMC o'lchovidan oldin harorat yoki boshqa boshqariladigan o'zgaruvchilar o'zgarganda stavka jarayoni bir xil bo'ladi deb taxmin qilishning hojati yo'q.
Oddiy
Eksperimental o'zgaruvchilarning (masalan, dastlabki kontsentratsiyalar) tezlik jarayonlariga ta'sirini taqqoslash uchun IMC kimyoviy va boshqa tahlil usullarini ishlab chiqishni va ishlatishni talab qilmaydi. Agar mutlaq ma'lumot kerak bo'lsa (masalan, jarayon natijasida ishlab chiqarilgan mahsulot miqdori), unda tahlillar parallel ravishda IMC uchun ishlatilgan namunalarda (va / yoki IMC ishlagandan keyin IMC namunalarida) o'tkazilishi mumkin. Natijada olingan ma'lumotlar IMC tomonidan olingan tezlik ma'lumotlarini kalibrlash uchun ishlatiladi.
Aralashmaslik
IMC tezlikni olish uchun markerlarni (masalan, lyuminestsent yoki radioaktiv moddalar) qo'shishni talab qilmaydi. Aralashtirilmagan namunalardan foydalanish mumkin va IMC ishga tushirilgandan so'ng namuna o'zgarmaydi (sodir bo'lgan jarayonlar bundan mustasno). IMCdan keyingi namuna har qanday fizikaviy, kimyoviy, morfologik yoki boshqa turdagi qiziqishlarga duch kelishi mumkin.
Ogohlantirishlar
O'tkazib yuborilgan ma'lumotlar
Metodik tavsifda ko'rsatilgandek, muhrlangan ampulani kiritish uchun IMC usuli qo'llanilganda, birinchi navbatda issiqlik oqimini olish mumkin emas. Namuna asta-sekin belgilangan haroratga keltirilganida 40 daqiqa. Shuning uchun ushbu rejimda IMC sekin boshlanadigan yoki ma'lum bir haroratda sekin sodir bo'ladigan jarayonlarni o'rganishga eng mos keladi. Ushbu ogohlantirish vaqtga ham tegishli oldin kiritish - ya'ni. namunani tayyorlash (unda tezlik jarayoni boshlanishi mumkin) va IMC qo'shish jarayonini boshlash o'rtasida vaqt o'tgan (Charlebois va boshq. 2003).[11] IMC uchun tanlangan harorat namuna tayyorlangan haroratdan (masalan, 25 ° C) sezilarli darajada yuqori bo'lsa (masalan, 37 ° C), bu oxirgi ta'sir odatda minimallashtiriladi.
Tashqi ma'lumotlar
IMC ushlaydi yig'ma namuna ichidagi barcha jarayonlardan kelib chiqadigan issiqlik ishlab chiqarish yoki iste'mol, shu jumladan
- Namuna ampulasining o'zi fizik-kimyoviy holatidagi mumkin bo'lgan o'zgarishlar; masalan. metall tarkibiy qismlarida stressni yumshatish, polimer komponentlarning oksidlanishi.
- Tirik hujayralarning metabolizmi va o'sishi o'rganilayotgan madaniy muhitning degradatsiyasi.
Shunday qilib, sodir bo'lishi mumkin bo'lgan barcha jarayonlarni aniqlash uchun eksperimental rejalashtirish va loyihalashga katta e'tibor berish kerak. Ko'pincha bir nechta jarayonlar sodir bo'ladimi yoki yo'q bo'lsa, ularning issiqlik oqimiga qo'shadigan hissasini muntazam ravishda aniqlash uchun mo'ljallangan dastlabki tadqiqotlarni ishlab chiqish va o'tkazish zarur. Tashqi issiqlik oqimi ma'lumotlarini yo'q qilish uchun strategiyalardan biri shundaki, foiz stavkasi jarayoni sodir bo'lgan namuna uchun issiqlik oqimini taqqoslash, qiziqish namunasidagi hamma narsani o'z ichiga olgan bo'sh namunadan tashqari, qiziqish namunasi bundan mustasno. foiz stavkasi jarayonidan o'tish. Bunga to'g'ridan-to'g'ri ikki ampula o'rtasida issiqlik oqimining farqi to'g'risida xabar beruvchi dubleks IMC modullariga ega asboblar yordamida erishish mumkin.
Ilovalar
IMC dasturlari ma'lumotlarining ba'zi bir maxsus manbalarini muhokama qilgandan so'ng, IMC tezligini tahlil qilishning bir nechta o'ziga xos toifalari qamrab olindi va har bir toifadagi so'nggi misollar (adabiyotlar bilan) muhokama qilindi.
IMC dastur ma'lumotlarining maxsus manbalari
Qo'llanmalar
The Bibliografiya Termal tahlil va kalorimetriya qo'llanmasining to'rtta keng jildini ro'yxati: Vol. 1 tamoyillar va amaliyot (1998), jild. 2 Noorganik va turli xil materiallarga arizalar (2003), jild. 3 Polimerlar va plastmassalarga qo'llanmalar (2002) va jild. 4 Makromolekulalardan Insonga (1999). Ular IMC dasturlari va taxminan oldin nashr etilgan misollar haqida ma'lumotning asosiy manbasini tashkil etadi (va adabiyotga havolalar). 2000 yil.
Arizalar
Ba'zi IMC asbob ishlab chiqaruvchilari dastur yozuvlarini yig'dilar va ularni ommaga taqdim etishdi. Eslatmalar ko'pincha (lekin har doim ham emas) jurnal qog'ozlarini moslashtirishdir. Bunga misol sifatida Mikrokalorimetriya kompendiumi Vol. TA Instruments, Inc. tomonidan taqdim etilgan va Bibliografiya.
"Oqsillar" jildning birinchi bo'limlari. Men bu erda qiziq emasman, chunki u ish bilan ta'minlangan tadqiqotlarni tavsiflaydi Izotermik titrlash kalorimetri. Volning keyingi bo'limlari. Men, hayot va biologik fanlar va farmatsevtika vositalarida IMC va Differentsial skanerlash kalorimetri. Vol. Kompendiumning II qismi deyarli to'liq IMC dasturlariga bag'ishlangan. Uning bo'limlari Tsement, Energetika, Materiallar va boshqalar deb nomlangan. Ushbu ikkita o'ziga xos kompendianing mumkin bo'lgan kamchiliklari shundaki, eslatmalarning hech biri eskirgan emas. Kompendiya 2009 yilda nashr etilgan bo'lsa-da, ba'zi eslatmalar bir necha yil oldin ishlatilgan va endi mavjud bo'lmagan IMC asboblarini tavsiflaydi. Shunday qilib, ba'zi bir eslatmalar, hali ham dolzarb va ibratli bo'lishiga qaramay, ko'pincha 2000 yilgacha olib borilgan tadqiqotlarni tasvirlaydi.
Ilovalarga misollar
Umuman olganda, IMC-ning mumkin bo'lgan dasturlari faqat IMC-ni analitik vosita sifatida ishlatishni tanlagan kishining tasavvurlari bilan cheklangan - mavjud IMC asboblari va metodikasi tomonidan ilgari tavsiflangan cheklovlar doirasida. Buning sababi shundaki, u har qanday kimyoviy, fizikaviy yoki biologik tezlikni kuzatish uchun universal vosita hisoblanadi. Quyida har birida misollar keltirilgan ba'zi IMC dasturlari toifalari keltirilgan. Ko'pgina toifalarda, eslatib o'tilgan va havolalarga qaraganda ko'proq nashr etilgan misollar mavjud. Kategoriyalar biroz o'zboshimchalik bilan va ko'pincha bir-biriga to'g'ri keladi. Boshqa toifadagi toifalar mantiqiy bo'lishi mumkin va ko'proq toifalar qo'shilishi mumkin.
Qattiq materiallar
Shakllanish
IMC turli xil jarayonlarni turli xil materiallarni shakllantirish tezligini o'rganish uchun keng qo'llaniladi. Sekin-asta yuzaga keladigan jarayonlarni o'rganish uchun eng mos keladi, ya'ni. soat yoki kun davomida. Bunga eng yaxshi misol - kaltsiy mineral tsement formulalarini gidratlash va sozlash reaktsiyalarini o'rganish. Bitta maqolada umumiy nuqtai nazar berilgan (Gawlicki va boshq. 2010)[12] boshqasida esa oddiy yondashuv tasvirlangan (Evju 2003).[13] Boshqa tadqiqotlar IMC tomonidan taqdim etilgan tsement gidratatsiyasi haqidagi tushunchalarga qaratilgan IQ spektroskopiyasi (Ylmen va boshq. 2010)[14] va kompozitsion o'zgaruvchilarning tsement gidratatsiyasiga va o'rnatish vaqtiga ta'sirini o'rganish uchun IMC-dan foydalanish to'g'risida (Xu va boshq. 2011).[15]
IMC kaltsiy minerallari yoki boshqa minerallarning gidratlanish darajasi va miqdorini (ma'lum namlik havosida) o'rganish uchun ham qulay foydalanish mumkin. Bunday tadqiqotlar uchun ma'lum namlik havosini ta'minlash uchun kichik miqdordagi to'yingan tuz eritmalarini IMC ampulasiga namlanmagan mineral namunasi bilan birga joylashtirish mumkin. Keyin ampula muhrlanadi va IMC asbobiga kiritiladi. To'yingan tuz eritmasi ampuladagi havoni ma'lum rH darajasida ushlab turadi va turli xil oddiy tuz eritmalari namlikni ta'minlaydi. 32-100% rH. Bunday tadqiqotlar mikronometr oralig'ida amalga oshirildi kaltsiy gidroksiapatit zarralar va kaltsiy o'z ichiga olgan bioaktiv shisha "nano" zarralari (Doostmohammadi va boshq. 2011).[16]
Barqarorlik
IMC materiallarning sekin o'zgarishi tezligini tezda aniqlash uchun juda mos keladi (Willson va boshq. 1995).[17] Bunday baholash turlicha, tanazzulga uchragan yoki saqlash muddati.

Shakl.4
For example, IMC has been widely used for many years in shelf life studies of solid drug formulations in the pharmaceutical industry (Pikal et al. 1989,[18] Hansen va boshq. 1990 yil,[19] Konigbauer et al. 1992 yil.[20]) IMC has the ability to detect slow degradation during simulated shelf storage far sooner than conventional analytical methods and without the need to employ chemical assay techniques. IMC is also a rapid, sensitive method for determining the often functionally crucial amorphous content of drugs such as nifedipine (Vivoda et al. 2011).[21]
IMC can be used for rapidly determining the rate of slow changes in industrial polymers. For example, gamma radiation sterilization of a material frequently used for surgical implants—ultra yuqori molekulyar og'irlikdagi polietilen (UHMWPE)—is known to produce free radicals in the polymer. The result is slow oxidation and gradual undesirable embrittlement of the polymer on the shelf or in vivo. IMC could detect oxidation-related heat and quantified an oxidation rate of ca. 1% per year in irradiated UHMWPE at room temperature in air (Charlebois et al. 2003).[11] In a related study the activation energy was determined from measurements at a series of temperatures (Hardison et al. 2003).[2]
IMC is also of great utility in evaluating the "runaway potential" of materials which are significant fire or explosion hazards. For example, it has been used to determine autocatalytic kinetics of kumen gidroperoksidi (CHP), an intermediate which is used in the chemical industry and whose sudden decomposition has caused a number of fires and explosions. Fig. 4 Shows the IMC data documenting thermal decomposition of CHP at 5 different temperatures (Chen et al. 2008).[22]
Biologiya va tibbiyot
The term metabolismics can be used[iqtibos kerak ] to describe studies of the quantitative measurement of the rate at which heat is produced or consumed vs. time by cells (including microbes) in culture, by tissue specimens, or by small whole organisms. As described subsequently, metabolismics can be useful as a diagnostic tool; especially in either (a) identifying the nature of a specimen from its heat flow vs. time signature under a given set of conditions, or (b) determining the effects of e.g. pharmaceutical compounds on metabolic processes, organic growth or viability. Metabolismics is related to metabolomika. The latter is the systematic study of the unique chemical fingerprints that specific cellular processes leave behind; i.e. the study of their small-molecule metabolite profiles. When IMC is used to determine metabolismics, the products of the metabolic processes studied are subsequently available for metabolomics studies. Since IMC does not employ biochemical or radioactive markers, the post-IMC specimens consist only of metabolic products and remaining culture medium (if any was used). If metabolismics and metabolomics are used together, they can provide a comprehensive record of a metabolic process taking place in vitro: its rate and energetics, and its metabolic products.
To determine metabolismics using IMC, there must of course be sufficient cells, tissue or organisms initially present (or present later if replication is taking place during IMC measurements) to generate a heat flow signal above a given instrument's detection limit. A landmark 2002 general paper on the topic of metabolism provides an excellent perspective from which to consider IMC metabolismic studies (see Bibliografiya, West, Woodruff and Brown 2002). It describes how metabolic rates are related and how they scale over the entire range from "molecules and mitochondria to cells and mammals". Importantly for IMC, the authors also note that while the metabolic rate of a given type of mammalian cell in vivo declines markedly with increasing animal size (mass), the size of the donor animal has no effect on the metabolic rate of the cell when cultured in vitro.
Cell and tissue biology
Mammalian cells in culture have a metabolic rate of ca. 30×10−12 W/cell (Figs. 2 and 3 in Bibliography: West, Woodruff and Brown 2002 ). By definition, IMC instruments have a sensitivity of at least 1×10−6 W (i.e. 1 μW). Therefore, the metabolic heat of ca. 33,000 cells is detectable. Based on this sensitivity, IMC was used to perform a large number of pioneering studies of cultured mammalian cell metabolismics in the 1970s and 1980s in Sweden. One paper (Monti 1990)[23] serves as an extensive guide to work done up until 1990. It includes explanatory text and 42 references to IMC studies of heat flow from cultured human eritrotsitlar, trombotsitlar, limfotsitlar, lymphoma cells, granulotsitlar, adipotsitlar, skeletal muscle, and myocardial tissue. The studies were done to determine how and where IMC might be used as a clinical diagnostic method and/or provide insights into metabolic differences between cells from healthy persons and persons with various diseases or health problems.
Developments since ca. 2000 in IMC (e.g. massively parallel instruments, real-time, computer-based storage and analysis of heat flow data) have stimulated further use of IMC in cultured cell biology. For example, IMC has been evaluated for assessing antigen-induced lymphocyte proliferation (Murigande et al. 2009)[24] and revealed aspects of proliferation not seen using a conventional non-continuous radioactive marker assay method. IMC has also been applied to the field of to'qima muhandisligi. One study (Santoro et al. 2011)[25] demonstrated that IMC could be used to measure the growth (i.e. proliferation) rate in culture of human xondrositlar harvested for tissue engineering use. It showed that IMC can potentially serve to determine the effectiveness of different growth media formulations and also determine whether cells donated by a given individual can be grown efficiently enough to consider using them to produce engineered tissue.
IMC has also been used to measure the metabolic response of cultured makrofaglar to surgical implant wear debris. IMC showed that the response was stronger to μm size range particles of polyethylene than to similarly sized Co alloy particles (Charlebois et al. 2002).[26] A related paper covers the general topic of applying IMC in the field of synthetic solid materials used in surgery and medicine (Lewis and Daniels 2003).[10]
At least two studies have suggested IMC can be of substantial use in tumor pathology. In one study (Bäckman 1990),[27] the heat production rate of T-lymphoma cells cultured in suspension was measured. Changes in temperature and pH induced significant variations, but stirring rate and cell concentration did not. A more direct study of possible diagnostic use (Kallerhoff et al. 1996)[28] produced promising results. For the uro-genital tissue biopsy specimens studied, the results showed
"it is possible to differentiate between normal and tumorous tissue samples by microcalorimetric measurement based on the distinctly higher metabolic activity of malignant tissue. Furthermore, microcalorimetry allows a differentiation and classification of tissue samples into their histological grading."
Toksikologiya
As of 2012, IMC has not become widely used in cultured cell toxicology even though it has been used periodically and successfully since the 1980s. IMC is advantageous in toxicology when it is desirable to observe cultured cell metabolism in real time and to quantify the rate of metabolic decline as a function of the concentration of a possibly toxic agent. One of the earliest reports (Ankerst et al. 1986)[29] of IMC use in toxicology was a study of antibody-dependent cellular toxicity (ADCC) against human melanoma cells of various combinations of antiserum, monoclonal antibodies and also peripheral blood lymphocytes as effector cells. Kinetics of melanoma cell metabolic heat flow vs. time in closed ampoules were measured for 20 hours. Mualliflar shunday xulosaga kelishdi
"...microcalorimetry is a sensitive and particularly suitable method for the analysis of cytotoxicity kinetics."
IMC is also being used in environmental toxicology. In an early study (Thorén 1992)[30] toxicity against monolayers of alveolar macrophages of particles of MnO2, TiO2 va SiO2 (silica) were evaluated. IMC results were in accord with results obtained by fluorescein ester staining and microscopic image analysis—except that IMC showed toxic effects of quartz not discernable by image analysis. This latter observation—in accord with known alveolar effects—indicated to the authors that IMC was a more sensitive technique.

Much more recently (Liu et al. 2007),[31] IMC has been shown to provide dynamic metabolic data which assess toxicity against fibroblasts of Cr(VI) from potassium chromate. Fig. 5 shows baseline results determining the metabolic heat flow from cultured fibroblasts prior to assessing the effects of Cr(VI). Mualliflar shunday xulosaga kelishdi
"Microcalorimetry appears to be a convenient and easy technique for measuring metabolic processes...in...living cells. As opposed to standard bioassay procedures, this technique allows continuous measurements of the metabolism of living cells. We have thus shown that Cr(VI) impairs metabolic pathways of human fibroblasts and particularly glucose utilization."
Simple closed ampoule IMC has also been used and advocated for assessing the cultured cell toxicity of candidate surgical implant materials—and thus serve as a biocompatibility screening method. In one study (Xie et al. 2000)[32] porcine renal tubular cells in culture were exposed to both polymers and titanium metal in the form of "microplates" having known surface areas of a few cm2. The authors concluded that IMC
"...is a rapid method, convenient to operate and with good reproducibility. The present method can in most cases replace more time-consuming light and electron microscopic investigations for quantitating of adhered cells."
In another implant materials study (Doostmohammadi et al. 2011)[33] both a rapidly growing yeast culture and a human chondrocyte culture were exposed to particles (diam.< 50 μm) of calcium hydroxyapatite (HA) and bioactive (calcium-containing) silica glass. The glass particles slowed or curtailed yeast growth as a function of increasing particle concentration. The HA particles had much less effect and never entirely curtailed yeast growth at the same concentrations. The effects of both particle types on chondrocyte growth were minimal at the concentration employed. Mualliflar shunday xulosaga kelishdi
"The cytotoxicity of particulate materials such as bioactive glass and hydroxyapatite particles can be evaluated using the microcalorimetry method. This is a modern method for in vitro study of biomaterials biocompatibility and cytotoxicity which can be used alongside the old conventional assays."
Mikrobiologiya

Publications describing use of IMC in microbiology began in the 1980s (Jesperson 1982).[34] While some IMC microbiology studies have been directed at viruses (Heng et al. 2005)[35] and fungi (Antoci et al. 1997),[36] most have been concerned with bacteria. A recent paper (Braissant et al. 2010)[37] provides a general introduction to IMC metabolismic methods in microbiology and an overview of applications in medical and environmental microbiology. The paper also explains how heat flow vs. time data for bacteria in culture are an exact expression—as they occur over time—of the fluctuations in microorganism metabolic activity and replication rates in a given medium (Fig. 6).

In general, bacteria are about 1/10 the size of mammalian cells and produce perhaps 1/10 as much metabolic heat-i.e. taxminan 3x10−12 W/cell. Thus, compared to mammalian cells (see above) ca. 10X as many bacteria—ca. 330,000—must be present to produce detectable heat flow—i.e. 1 μW.[37] However, many bacteria replicate orders of magnitude more rapidly in culture than mammalian cells, often doubling their number in a matter of minutes (see Bacterial growth ). As a result, a small initial number of bacteria in culture and initially undetectable by IMC rapidly produce a detectable number. For example, 100 bacteria doubling every 20 minutes will in less than 4 hours produce >330,000 bacteria and thus an IMC-detectable heat flow. Consequently, IMC can be used for easy, rapid detection of bacteria in the medical field. Examples include detection of bacteria in human blood platelet products (Trampuz et al. 2007)[38] and urine (Bonkat et al. 2011)[39] and rapid detection of tuberculosis (Braissant et al. 2010,[40] Rodriguez va boshq. 2011 yil[41]). Fig. 7 shows an example of detection times of sil kasalligi bacteria as a function of the initial amount of bacteria present in a closed IMC ampoule containing a culture medium.
For microbes in growth media in closed ampoules, IMC heat flow data can also be used to closely estimate basic microbial growth parameters; i.e. maximum growth rate and duration time of the lag phase before maximum growth rate is achieved. This is an important special application of the basic analysis of these parameters explained previously (Overview: Data Obtained ).
Unfortunately, the IMC literature contains some published papers in which the relation between heat flow data and microbial growth in closed ampoules has been misunderstood. However, in 2013 an extensive clarification was published, describing (a) details of the relation between IMC heat flow data and microbial growth, (b) selection of mathematical models which describe microbial growth and (c) determination of microbial growth parameters from IMC data using these models (Braissant et al. 2013).[42]
Farmakodinamika
In a logical extension of the ability of IMC to detect and quantify bacterial growth, known concentrations of antibiotics can be added to bacterial culture, and IMC can then be used to quantify their effects on viability and growth. Closed ampoule IMC can easily capture basic pharmacologic information—e.g. minimum inhibitory concentration (MIC) of an antibiotic needed to stop growth of a given organism. In addition it can simultaneously provide dynamic growth parameters—lag time and maximum growth rate (see Fig. 2, Howell et al. 2011, Braissant et al. 2013),[1][42] which assess mechanisms of action. Bactericidal action (see Bakteritsid ) is indicated by an increased lag time as a function of increasing antibiotic concentration, while bacteriostatic action (see Bakteriostatik vosita ) is indicated by a decrease in growth rate with concentration. The IMC approach to antibiotic assessment has been demonstrated for a number of a types of bacteria and antibiotics (von Ah et al. 2009).[43] Closed ampoule IMC can also rapidly differentiate between normal and resistant strains of bacteria such as Staphylococcus aureus (von Ah et al. 2008,[44] Baldoni et al. 2009 yil[45]). IMC has also been used to assess the effects of disinfectants on the viability of mouth bacteria adhered to dental implant materials (Astasov-Frauenhoffer et al. 2011).[46] In a related earlier study, IMC was used to measure the heat of adhesion of dental bacteria to glass (Hauser-Gerspach et al. 2008).[47]
Analogous successful use of IMC to determine the effects of antitumor drugs on tumor cells in culture within a few hours has been demonstrated (Schön and Wadsö 1988).[48] Rather than the closed-ampoule approach, an IMC setup was used which allowed drug injection into stirred specimens.
As of 2013, IMC has been used less widely in mammalian cell in vitro pharmacodynamic studies than in microbial studies.
Ko'p hujayrali organizmlar
It is possible to use IMC to perform metabolismic studies of living multicellular organisms—if they are small enough to be placed in IMC ampoules (Lamprecht & Becker 1988).[49] IMC studies have been made of insect pupa metabolism during ventilating movements (Harak et al. 1996)[50] and effects of chemical agents on pupal growth (Kuusik et al. 1995).[51] IMC has also proved effective in assessing the effects of aging on nematode worm metabolism (Braekman et al. 2002).[52]
IMC has also proved highly useful for in vitro assessments of the effects of pharmaceuticals on tropical parasitic worms (Manneck et al. 2011-1,[53] Maneck et al. 2011-2,[9] Kirchhofer et al. 2011).[54] An interesting feature of these studies is the use of a simple manual injection system for introducing the pharmaceuticals into sealed ampoules containing the worms. Also, IMC not only documents the general metabolic decline over time due to the drugs, but also the overall frequency of worm motor activity and its decline in amplitude over time as reflected in fluctuations in the heat flow data.
Atrof-muhit biologiyasi
Because of its versatility, IMC can be an effective tool in the fields of plant and environmental biology. In an early study (Hansen et al. 1989),[55] the metabolic rate of larch tree clone tissue specimens was measured. The rate was predictive of long-term tree growth rates, was consistent for specimens from a given tree and was found to correlate with known variations in the long-term growth of clones from different trees.
Bacterial oxalotrophic metabolism is common in the environment, particularly in soils. Oxalotrophic bacteria are capable of using oxalate as a sole carbon and energy source. Closed-ampoule IMC was used to study metabolism of oxalotrophic soil bacteria exposed to both an optimized medium containing potassium oxalate as the sole carbon source and a model soil (Bravo et al. 2011).[56] Using an optimized medium, growth of six different strains of soil bacteria was easily monitored and reproducibly quantified and differentiated over a period days. IMC measurement of bacterial metabolic heat flow in the model soil was more difficult, but a proof of concept was demonstrated.
Oy suti is a white, creamy material found in caves. It is a non-hardening, fine crystalline precipitate from limestone and is composed mainly of calcium and/or magnesium carbonates. Microbes may be involved in its formation. It is difficult to infer microbial activities in moonmilk from standard static chemical and microscopic assays of moonmilk composition and structure. Closed ampoule IMC has been used to solve this problem (Braissant, Bindscheidler et al. 2011).[57] It was possible to determine the growth rates of chemoheterotrophic microbial communities on moonmilk after the addition of various carbon sources simulating mixes that would be brought into contact with moonmilk due to snow melt or rainfall. Metabolic activity was high and comparable to that found in some soils.
Harris et al. (2012),[58] studying differing fertilizer input regimes, found that, when expressed as heat output per unit soil microbial biomass, microbial communities under organic fertilizer regimes produced less waste heat than those under inorganic regimes.
Oziq-ovqat fani
IMC has been shown to have diverse uses in oziq-ovqat fanlari va texnologiya. An overview (Wadsö and Galindo 2009)[59] discusses successful applications in assessing vegetable cutting wound respiration, cell death from blanching, milk fermentation, microbiological spoilage prevention, thermal treatment and shelf life. Another publication (Galindo et al. 2005)[60] reviews the successful use of IMC for monitoring and predicting quality changes during storage of minimally processed fruits and vegetables.
IMC has also proven effective in accomplishing enzymatic assays for orotic acid in milk (Anastasi et al. 2000)[61] va molik kislota in fruits, wines and other beverages and also cosmetic products (Antonelli et al. 2008).[62] IMC has also been used to assess the efficacy of anti-browning agents on fresh-cut potatoes (Rocculi et al. 2007).[63] IMC has also proven effective in assessing the extent to which low-energy pulsed electric fields (PEFs) affect the heat of nihol of barley seeds—important in connection with their use in producing malted beverages (Dymek et al. 2012).[64]
Shuningdek qarang
- Kalorimetriya
- Kimyoviy termodinamika
- Differentsial skanerlash kalorimetri
- Izotermik titrlash kalorimetri
- Rate equation
- Termoelektrik ta'sir
Bibliografiya
- Harris, JA; Ritz, K; Coucheney, E; Grice, SM; Lerch, TZ; Pawlett, M; Herrmann, AM (2012). "The thermodynamic efficiency of soil microbial communities subject to long-term stress is lower than those under conventional input regimes". Tuproq biologiyasi va biokimyo. 47: 149–157. doi:10.1016/j.soilbio.2011.12.017.
- Glasstone S, Laidler KJ, Eyring H (1941) The theory of rate processes: the kinetics of chemical reactions, viscosity, diffusion and electrochemical phenomena. McGraw-Hill (New York). 611p.
- Johnson FH, Eyring H, Stover BJ (1974) The theory of rate processes in biology and medicine. Wiley (New York), ISBN 0-471-44485-5, 703p.
- Lavoisier A & Laplace PS (1780) M´emoire sur la chaleur. Académie des Sciences, Paris.
- Brown ME, Editor (1998) Vol. 1 Principles and Practice (691p.), in Handbook of Thermal Analysis and Calorimetry. Gallagher PK (Series Editor). Elsevier (London).
- Brown ME and Gallagher PK, Editors (2003) Vol. 2 Applications to Inorganic and Miscellaneous Materials (905p.), in Handbook of Thermal Analysis and Calorimetry. Gallagher PK (Series Editor). Elsevier (London). ISBN 978-0-444-82086-0
- Cheng SZD, Editor (2002) Vol. 3 Applications to Polymers and Plastics (828p.) in Handbook of Thermal Analysis and Calorimetry. Gallagher PK (Series Editor). Elsevier (London).
- Kemp RB, Editor (1999) Vol. 4 From Macromolecules to Man (1032p.), in Handbook of Thermal Analysis and Calorimetry. Gallagher PK (Series Editor). Elsevier (London).
- Microcalorimetry Compendium Vol. 1: Proteins, Life & Biological Sciences, Pharmaceuticals (2009). TA Instruments, Inc. (New Castle DE, USA).
- Microcalorimetry Compendium Vol. 2: Cement, Energetics, Material, Other (2009). TA Instruments, Inc. (New Castle DE, USA).
- West, GB; Woodruff, WH; Brown, JH (2002). "Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals". PNAS. 99: 2473–2478. Bibcode:2002PNAS...99.2473W. doi:10.1073/pnas.012579799. PMC 128563. PMID 11875197.
Adabiyotlar
- ^ a b Xauell, M; Wirz D; Daniels AU; Braissant O (November 2011). "Application of a microcalorimetric method for determining drug susceptibility in Mycobacterium species". Klinik mikrobiologiya jurnali. 50 (1): 16–20. doi:10.1128/JCM.05556-11. PMC 3256699. PMID 22090404.
- ^ a b v Hardison, A; Lewis GW; Daniels AU (2003). "Determination of the activation energies of and aggregate rates for exothermic physico-chemical changes in UHMWPE by isothermal heat-conduction microcalorimetry (IHCMC)". Biyomateriallar. 24 (28): 5145–5151. doi:10.1016/S0142-9612(03)00461-7. PMID 14568431.
- ^ Wadsö, L (1968). "Design and testing of a microreaction calorimeter" (PDF). Acta Chemica Scandinavica. 22: 927–937. doi:10.3891/acta.chem.scand.22-0927.
- ^ Suurkuusk, J; Wadsö, L (1974). "Design and testing of an improved precise drop calorimeter for the measurement of heat capacity of small samples". J. Chem. Termodinamika. 6 (7): 667–679. doi:10.1016/0021-9614(74)90117-7.
- ^ Suurkuusk, J (1982). "A multichannel microcalorimetry system". Kimyoviy. Scr. 20: 155–163.
- ^ Thorén, SA; Suurkuusk J; Holma B (1989). "Operation of a multichannel microcalorimetry system in the micro-submicrowatt region: some methodological aspects". Biokimyoviy va biofizik usullar jurnali. 18 (2): 149–156. doi:10.1016/0165-022X(89)90076-6. PMID 2745930.
- ^ a b v d Wadsö, I; Goldberg, RN (2001). "Standards in isothermal microcalorimetry". Sof Appl. Kimyoviy. 73 (10): 1625–1639. doi:10.1351/pac200173101625. S2CID 44976071.
- ^ van Herwaarden S (2000) Calorimetry measurement. in: MechanicalVariables Measurement (Webster JG, ed), pp. 17.1–17.16. CRC Press, Boka Raton, Florida.
- ^ a b Manneck, T; Braissant O; Haggenmueller Y; Keiser J (2011). "Isothermal Microcalorimetry To Study Drugs against Schistosoma mansoni". Klinik mikrobiologiya jurnali. 49 (4): 1217–1225. doi:10.1128/JCM.02382-10. PMC 3122815. PMID 21270220.
- ^ a b Lewis, G; Daniels AU (2003). "Use of Isothermal Heat-Conduction Microcalorimetry (IHCMC) for the Evaluation of Synthetic Biomaterials". J. Biomed. Materials Res.-B. 66B (2): 487–501. CiteSeerX 10.1.1.517.6452. doi:10.1002/jbm.b.10044. PMID 12861599.
- ^ a b Charlebois, SJ; Daniels AU; Lewis G (2003). "Isothermal Microcalorimetry: An Analytical Technique for Assessing the Dynamic Chemical Stability of UHMWPE". Biyomateriallar. 24 (2): 91–296. doi:10.1016/S0142-9612(02)00317-4. PMID 12419630.
- ^ Gawlicki, M; Nocun-Wczelik, W; Bak, L (2010). "Calorimetry in the studies of cement hydration". J Therm Anal Calorim. 100 (2): 571–6. doi:10.1007/s10973-009-0158-5. S2CID 137241273.
- ^ Evju, C (2003). "Initial hydration of cementitious systems using a simple isothermal calorimeter and dynamic correction". J Therm Anal Calorim. 71 (3): 829–40. doi:10.1023/A:1023374125778. S2CID 93452683.
- ^ Ylmen, R; Wadso, L; Panas, I (2010). "Insights into early hydration of Portland limestone cement from infrared spectroscopy and isothermal calorimetry". Cem Concr Res. 40 (10): 1541–6. doi:10.1016/j.cemconres.2010.06.008.
- ^ Xu L, Wang P, Zhang G (2011) Calorimetric study on the influence of calcium sulfate on the hydration of Portland cement-calcium aluminate cement mixtures. J. Thermal Analysis and Calorimetry (pub. on line 5 October 2011).
- ^ Doostmohammadi, A; Monshi, A; Fathi, MA; Braissant, O (2011). "A comparative physico-chemical study of bioactive glass and bone-derived hydroxyapatite". Ceramic International. 37 (5): 1601–1607. doi:10.1016/j.ceramint.2011.03.009.
- ^ Willson, RJ; Beezer, AE; Mitchell, JC; Loh, W (1995). "Determination of thermodynamic and kinetic parameters from isothermal heat conduction microcalorimetry: applications to long term reaction studies". J. Fiz. Kimyoviy. 99 (18): 7108–7113. doi:10.1021/j100018a051.
- ^ Pikal, MJ; Dellerman, KM (1989). "Stability testing of pharmaceuticals by high-sensitivity isothermal calorimetry at 25°C: cephalosporins in the solid and aqueous solution states". Int J Pharmacol. 50 (3): 233–252. doi:10.1016/0378-5173(89)90127-0.
- ^ Xansen, LD; Eatough, DJ; Lewis, EA; Bergstrom, RG; Degraft-Johnson, D; Cassidy-Thompson, K (1990). "Shelf-life prediction from induction period calorimetric measurements on materials undergoing autocatalytic decomposition". Kanada kimyo jurnali. 68 (11): 2111–2114. doi:10.1139/v90-321.
- ^ Koenigbauer, MJ; Brooks SH; Rullo G; Couch RA (1992). "Solid-state stability testing of drugs by isothermal calorimetry". Farmatsevtika tadqiqotlari. 9 (7): 933–44. doi:10.1023/a:1015865319250. PMID 1438010. S2CID 12884493.
- ^ Vivoda, M; Roskar, R; Kmetec, V (2011). "The development of a quick method for amorphicity determination by isothermal microcalorimetry". J Therm Anal Calorim. 105 (3): 1023–1030. doi:10.1007/s10973-011-1443-7. S2CID 95028157.
- ^ Chen, J-R; Wu, S-H; Lin, S-Y; Hou, H-Y; Shu, C-M (2008). "Utilization of Microcalorimetry for an Assessment of the Potential for a Runaway Decomposition of Cumene Hydroperoxide at Low Temperatures". J Therm Anal Calorim. 93 (1): 127–133. doi:10.1007/s10973-007-8834-9. S2CID 96305303.
- ^ Monti, M (1990). "Application of microcalorimetry to the study of living cells in the medical field". Thermochimica Acta. 172: 53–60. doi:10.1016/0040-6031(90)80558-g.
- ^ Murigande, C; Regenass S; Wirz D; Daniels AU; Tyndall A (2009). "A Comparison Between (3H)-thymidine Incorporation and Isothermal Microcalorimetry for the Assessment of Antigen-induced Lymphocyte Proliferation". Immunologik tekshiruvlar. 38 (1): 67–75. doi:10.1080/08820130802572160. PMID 19172486. S2CID 38795681.
- ^ Santoro, R; Braissant O; Müller B; Wirz D; Daniels A.U.; Martin I; Wendt D (2011). "Real-time measurements of human chondrocyte heat production during in vitro proliferation". Biotexnologiya va bioinjiniring. 108 (12): 3019–3024. doi:10.1002/bit.23268. PMID 21769860. S2CID 19299843.
- ^ Charlebois, SJ; Daniels AU; Smith RA (2002). "Metabolic Heat Production as a Measure of Macrophage Response to Particles from Orthopaedic Implant Materials". Biomedikal materiallarni tadqiq qilish jurnali. 59 (1): 166–175. doi:10.1002/jbm.1230. PMID 11745550.
- ^ Bäckman, P (1990). "Effects of experimental factors on the metabolic rate of t-lymphoma cells as measured by microcalorimetry". Thermochimica Acta. 172 (1): 123–130. doi:10.1016/0040-6031(90)80566-h.
- ^ Kallerhoff, M; Karnebogen M; Singer D; Dettenbaeh A; Gralher U; Ringert R-H (1996). "Microcalorimetric measurements carried out on isolated tumorous and nontumorous tissue samples from organs in the urogenital tract in comparison to histological and impulse-cytophotometric investigations". Urological Research. 24 (2): 83–91. doi:10.1007/bf00431084. PMID 8740977. S2CID 35744559.
- ^ Ankerst, J; Sjögren, HO; Fäldt, R (1986). "Use of microcalorimetry in analyzing the kinetics of ADCC". Journal of Immunological Research Methods. 88 (2): 259–264. doi:10.1016/0022-1759(86)90014-1. PMID 3958501.
- ^ Thorén, SA (1992). "Calorimetry: a new quantitative in vitro method in cell toxicology. A dose/effect study of alveolar macrophages exposed to particles". J Toksikol atrof-muhit salomatligi. 36 (4): 307–18. doi:10.1080/15287399209531641. PMID 1507265.
- ^ Liu, V.; Chaspoul, F.; Berge Lefranc, D.; Decome, L.; Gallice, P. (12 July 2007). "Microcalorimetry as a tool for Cr(VI) toxicity evaluation of human dermal fibroblasts". Journal of Thermal Analysis and Calorimetry. 89 (1): 21–24. doi:10.1007/s10973-006-7918-2. S2CID 96774590.
- ^ Xie, Y; Depierre JW; Nässberger LN (2000). "Biocompatibility of microplates for culturing epithelial renal cells evaluated by a microcalorimetric technique". Materialshunoslik jurnali: tibbiyotdagi materiallar. 11 (9): 587–591. doi:10.1023/A:1008984304821. PMID 15348389. S2CID 25818381.
- ^ Doostmohammadi, A; Monshi A; Fathi MH; Karbasi S; Braissant O; Daniels AU (2011). "Direct cytotoxicity evaluation of 63S bioactive glass and bone-derived hydroxyapatite particles using yeast model and human chondrocyte cells by microcalorimetry". Materialshunoslik jurnali: tibbiyotdagi materiallar. 22 (10): 2293–2300. doi:10.1007/s10856-011-4400-x. PMID 21786131. S2CID 25271308.
- ^ Jespersen ND (1982) Biochemical and Clinical Applications of Thermometric and Thermal Analysis. Elsevier Scientific Publishing Company, Amsterdam.
- ^ Heng, Z.; Congyi, Z.; Cunxin, W.; Jibin, W.; Chaojiang, G.; Jie, L.; Yuwen, L. (January 2005). "Microcalorimetric study of virus infection; The effects of hyperthermia and 1b recombinant homo interferon on the infection process of BHK-21 cells by foot and mouth disease virus". Journal of Thermal Analysis and Calorimetry. 79 (1): 45–50. doi:10.1007/s10973-004-0560-y. S2CID 98578017.
- ^ Antoce, O-A; Antocie, V; Takaxashi, K; Pomohaci, N; Namolosanu, I (1997). "Calorimetric determination of the inhibitory effect of C1-C4 n-alcohols on growth of some yeast species". Thermochimica Acta. 297 (1–2): 33–42. doi:10.1016/s0040-6031(97)00162-7.
- ^ a b Braissant, O.; Wirz, D.; Gopfert, B.; Daniels, A. U. (2010). "Use of isothermal microcalorimetry to monitor microbial activities". FEMS Mikrobiol. Lett. 303 (1): 1–8. doi:10.1111/j.1574-6968.2009.01819.x. PMID 19895644.
- ^ Trampuz, A; Salzmann S; Antheaume J; Daniels AU (2007). "Microcalorimetry: a novel method for detection of microbial contamination in platelet products". Qon quyish. 47 (9): 1643–1650. doi:10.1111/j.1537-2995.2007.01336.x. PMID 17725729. S2CID 21221691.
- ^ Bonkat, G; Braissant O; Widmer AF; Frei R; Rieken M; Wyler S; Gasser TC; Wirz D; Daniels AU; Bachmann A (2011). "Rapid detection of urinary tract pathogens using microcalorimetry: principle, technique and first results". British Journal of Urology International. 110 (6): 892–897. doi:10.1111/j.1464-410X.2011.10902.x. PMID 22313675. S2CID 34620719.
- ^ Braissant, O; Wirz D; Gopfert B; Daniels AU (2010). "The heat is on: rapid microcalorimetric detection of mycobacteria in culture". Tuberculosis (Edinb). 90 (1): 57–59. doi:10.1016/j.tube.2009.11.001. PMID 19969505.
- ^ Rodríguez, D; Daniels AU; Urrusti JL; Wirz D; Braissant O (October 2011). "Evaluation of a low-cost calorimetric approach for rapid detection of tuberculosis and other mycobacteria in culture". Amaliy mikrobiologiya jurnali. 111 (4): 1016–1024. doi:10.1111/j.1365-2672.2011.05117.x. PMID 21797951. S2CID 205324227.
- ^ a b Braissant, O; Bonkat, G; Wirz, D (2013). "Microbial growth and isothermal microcalorimetry: Growth models and their application to microcalorimetric data". Thermochimica Acta. 555: 64–71. doi:10.1016/j.tca.2012.12.005.
- ^ von Ah, U; Wirz D; Daniels AU (2009). "Isothermal micro calorimetry—a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus". BMC Mikrobiol. 9 (1): 106. doi:10.1186/1471-2180-9-106. PMC 2692853. PMID 19470161.
- ^ von Ah, U; Wirz D; Daniels AU (2008). "Rapid differentiation of methicillin-susceptible Staphylococcus aureus from methicillin-resistant S. aureus and MIC determinations by isothermal microcalorimetry". J Clin Microbiol. 46 (6): 2083–7. doi:10.1128/JCM.00611-08. PMC 2446841. PMID 18417657.
- ^ Baldoni, D; Hermann H; Frei R; Trampuz A; Steinhuber A (2009). "Performance of microcalorimetry for early detection of methicillin resistance in clinical isolates of Staphylococcus aureus". J Clin Microbiol. 47 (3): 774–776. doi:10.1128/JCM.02374-08. PMC 2650961. PMID 19158262.
- ^ Astasov-Frauenhoffer, M; Braissant O; Hauser-Gerspach I; Daniels AU; Wirz D; Weiger R; Waltimo T (2011). "Quantification of vital adherent Streptococcus sanguinis cells on protein-coated titanium after disinfectant treatment" (PDF). Materialshunoslik jurnali: tibbiyotdagi materiallar. 22 (9): 2045–2051. doi:10.1007/s10856-011-4377-5. PMID 21670995. S2CID 11255313.
- ^ Hauser-Gerspach, I; Scandiucci de Freitas P; Daniels AU; Meyer J (2008). "Adhesion of Streptococcus sanguinis to glass surfaces measured by isothermal microcalorimetry (IMC)". J Biomed Mater Res B. 85 (1): 42–9. doi:10.1002/jbm.b.30914. PMID 17696148.
- ^ Schön, Wadsö I (1988). "The potential use of microcalorimetry in predictive tests of the action of antineoplastic drugs on mammalian cells". Sitobios. 55 (220): 33–39. PMID 3265371.
- ^ Lamprecht, I; Becker, W (1988). "Combination of calorimetry and endoscopy for monitoring locomotor activities of small animals". Thermochimica Acta. 130: 87–93. doi:10.1016/0040-6031(88)87053-9.
- ^ Harak, M; Lamprecht, I; Kuusik, A (1996). "Metabolic cost of ventilating movements in pupae of Tenebrio molitor and Galleria mellonella studied by direct calorimetry". Thermochimica Acta. 276: 41–47. doi:10.1016/0040-6031(95)02750-5.
- ^ Kuusik, A; Harak, M; Hiiesaar, K; Metspalu, L; Tartes, U (1995). "Studies on insect growth regulating (IGR) and toxic effects of Ledum palustre extracts on Tenebrio molitor pupae (Coleoptera, Tenebrionidae) using calorimetric recordings". Thermochimica Acta. 251: 247–253. doi:10.1016/0040-6031(94)02048-s.
- ^ Braeckman, BP; Houthoofd K; De Vreese A; Vanfleteren JR (2002). "Assaying metabolic activity in ageing Caenorhabditis elegans". Qarish va rivojlanish mexanizmlari. 123 (2002): 105–119. doi:10.1016/S0047-6374(01)00331-1. PMID 11718805. S2CID 26024344.
- ^ Manneck, T; Braissant O; Ellis W; Keiser J (2011). "Schistosoma mansoni: Antischistosomal activity of the four optical isomers and the two racemates of mefloquine on schistosomula and adult worms in vitro and in vivo". Eksperimental parazitologiya. 127 (1): 260–269. doi:10.1016/j.exppara.2010.08.011. PMID 20732321.
- ^ Kirchhofer, C; Vargas M; Braissant O; Dong Y; Vang X; Vennerstrom JL; Keiser J (2011). "Activity of OZ78 analogues against Fasciola hepatica and Echinostoma caproni". Acta Tropica. 118 (1): 56–62. doi:10.1016/j.actatropica.2011.02.003. PMC 3066657. PMID 21316331.
- ^ Xansen, LD; Lewis, EA; Eatough, DJ; Fowler, DP; Criddle, RS (1989). "Prediction of long-term growth rates of larch clones by calorimetric measurement of metabolic heat rates". Kanada o'rmon tadqiqotlari jurnali. 19 (5): 606–611. doi:10.1139/x89-095.
- ^ Bravo, D; Braissant O; Solokhina A; Clerc M; Daniels AU; Verrecchia E; Junier P (2011). "Use of an isothermal microcalorimetry assay to characterize microbial oxalotrophic activity". FEMS Mikrobiologiya Ekologiyasi. 78 (2): 266–74. doi:10.1111/j.1574-6941.2011.01158.x. PMID 21696406.
- ^ Braissant O, Bindschedler S, Daniels AU, Verrecchia EP & Cailleau C (2011) "Microbiological activities in moonmilk monitored using isothermal microcalorimetry (cave of "Vers chez le Brandt", Neuchatel, Switzerland)". Journal of Cave and Karst studies (accepted 05/2011).
- ^ Harris, JA; Ritz, K; Coucheney, E; Grice, SM; Lerch, TZ; Pawlett, M; Herrmann, AM (2012). "The thermodynamic efficiency of soil microbial communities subject to long-term stress is lower than those under conventional input regimes". Tuproq biologiyasi va biokimyo. 47: 149–157. doi:10.1016/j.soilbio.2011.12.017.
- ^ Wadsö, L; Gomez Galindo, F (2009). "Isothermal calorimetry for biological applications in food science and technology". Oziq-ovqat mahsulotlarini nazorat qilish. 20 (10): 956–961. doi:10.1016/j.foodcont.2008.11.008.
- ^ Gomez Galindo, F; Rocculi, P; Wadsö, L; Sjöholm, I (2005). "The potential of isothermal calorimetry in monitoring and predicting quality changes during processing and storage of minimally processed fruits and vegetables". Trends Food Sci Technol. 16 (8): 325–331. doi:10.1016/j.tifs.2005.01.008.
- ^ Anastasi, G; Antonelli ML; Biondi B; Vinci G (2000). "Orotic acid: a milk constituent Enzymatic determination by means of a new microcalorimetric method". Talanta. 52 (5): 947–952. doi:10.1016/S0039-9140(00)00433-1. PMID 18968055.
- ^ Antonelli, ML; Spadaro C; Tornelli RF (2008). "A microcalorimetric sensor for food and cosmetic analyses: L-malic acid determination". Talanta. 74 (5): 1450–1454. doi:10.1016/j.talanta.2007.09.035. PMID 18371803.
- ^ Rocculi, P; Gomez Galindo, F; Mendozac, F; Wadsö, L; Romani, S; Dalla Rosa, M; Sjöholm, I (2007). "Effects of the application of anti-browning substances on the metabolic activity and sugar composition of fresh-cut potatoes". Terimdan keyingi biologiya va texnologiya. 43: 151–157. doi:10.1016/j.postharvbio.2006.08.002.
- ^ Dymek K, Dejmek P, Panarese V, Vicente AA, Wadsö L, Finnie C, Gómez Galindo F (2012) Effect of pulsed electric field on the germination of barley seeds. LWT - Food Science and Technology (accepted 12/2011).
Tashqi havolalar
- Some sources for IMC instruments, accessories, supplies, and software
- Calmetrix
- TA asboblari
- Setaram
- Symcel
- Flow Adsorption Microcalorimeter instrument configurations Microscal Ltd (archived 2005)
