Polimeraza zanjiri reaktsiyasi - Polymerase chain reaction

Polimeraza zanjiri reaktsiyasi (PCR) - aniq bir nusxani millionlab milliardlab nusxada tezkor ravishda yaratish uchun keng qo'llaniladigan usul DNK namuna, bu olimlarga DNKning juda kichik namunasini olish va uni batafsil o'rganish uchun etarlicha katta miqdorda oshirish imkonini beradi. PCR 1984 yilda ixtiro qilingan Amerika biokimyogar Kari Mullis da Cetus korporatsiyasi. Bu genetik testlarning ko'pchiligini, shu jumladan tahlillarini o'tkazish uchun muhimdir qadimgi DNK namunalari va yuqumli kasalliklarni aniqlash. PCR yordamida juda oz miqdordagi nusxalar DNK ketma-ketliklari harorat o'zgarishi tsikllarida eksponent ravishda kuchaytiriladi. PCR endi keng tarqalgan va ko'pincha ajralmas usuldir tibbiy laboratoriya va turli xil amaliy dasturlar uchun klinik laboratoriya tadqiqotlari biomedikal tadqiqotlar va jinoiy sud ekspertizasi.[1][2]
PCR usullarining aksariyati ishonadi issiqlik velosiped. Termal velosiped reaktivlarni har xil haroratga bog'liq reaktsiyalarni ta'minlash uchun takroriy isitish va sovutish davrlariga ta'sir qiladi, xususan, DNKning erishi va ferment - haydovchi DNKning replikatsiyasi. PCR ikkita asosiy reaktivni ishlatadi - astarlar (ular ma'lum bo'lgan qisqa DNKning DNK fragmentlari oligonukleotidlar bu a bir-birini to'ldiruvchi maqsadli DNK mintaqasiga ketma-ketlik) va a DNK polimeraza. PCR ning birinchi bosqichida DNK juft spiralining ikkita ipi yuqori haroratda jismonan ajratilgan deb nomlanadi. nuklein kislota denaturatsiyasi. Ikkinchi bosqichda harorat pasayadi va primerlar DNKning komplementar ketma-ketliklariga bog'lanadi. Keyin ikkita DNK zanjiri aylanadi andozalar DNK polimeraza uchun fermentativ ravishda yangi DNK zanjirini tekinga yig'ing nukleotidlar, DNKning qurilish bloklari. PCR rivojlanishi bilan hosil bo'lgan DNKning o'zi a harakatini o'rnatgan holda replikatsiya uchun shablon sifatida ishlatiladi zanjir reaktsiyasi unda asl DNK shablonidir eksponent sifatida kuchaytirilgan.
Deyarli barcha PCR dasturlari issiqqa chidamli DNK polimeraza, masalan Taq polimeraza, dastlab dan ajratilgan ferment termofil bakteriya Thermus aquaticus. Agar ishlatiladigan polimeraza issiqlikka sezgir bo'lsa, u denaturatsiya pog'onasining yuqori harorati ostida denaturatsiya qilinadi. Foydalanishdan oldin Taq polimeraza, DNK-polimeraza har tsiklda qo'lda qo'shilishi kerak edi, bu zerikarli va qimmat jarayon edi.[3]
Texnikaning qo'llanilishi quyidagilarni o'z ichiga oladi DNKni klonlash uchun ketma-ketlik, genlarni klonlash va manipulyatsiya, gen mutagenezi; DNK asosidagi qurilish filogeniyalar, yoki funktsional tahlil genlar; tashxis va monitoring ning irsiy kasalliklar; qadimiy DNKni kuchaytirish;[4] uchun genetik barmoq izlarini tahlil qilish DNKni profillash (masalan, ichida sud ekspertizasi va ota-ona sinovlari ); va aniqlash patogenlar yilda nuklein kislota sinovlari diagnostikasi uchun yuqumli kasalliklar.

Printsiplar


PCR DNK zanjirining ma'lum bir mintaqasini kuchaytiradi (DNK nishoni). Ko'pgina PCR usullari 0,1 dan 10 gacha bo'lgan DNK fragmentlarini kuchaytiradi kilo tayanch juftliklari (kbp) uzunlikda, garchi ba'zi texnikalar parchalarni 40 kbpgacha kuchaytirishga imkon beradi.[5] Kuchaytirilgan mahsulot miqdori reaktsiyada mavjud bo'lgan substratlar bilan belgilanadi, bu reaktsiya o'tishi bilan cheklovga aylanadi.[6]
PCRni sozlash uchun bir nechta komponentlar va reaktivlar kerak,[7] shu jumladan:
- a DNK shabloni Kuchaytirish uchun DNK maqsad mintaqasini o'z ichiga oladi
- a DNK polimeraza; bu ferment polimerlashadi yangi DNK zanjirlari; issiqqa chidamli Taq polimeraza ayniqsa keng tarqalgan,[8] chunki yuqori haroratli DNKni denatürasyon jarayonida buzilmasdan qolish ehtimoli yuqori
- ikkita DNK astarlar bu bir-birini to'ldiruvchi uchun 3 ′ (uchta asosiy) uchi har birining sezgi va hissiyotga qarshi DNK nishonining iplari (DNK polimeraza faqat DNKning ikki zanjirli mintaqasi bilan bog'lanishi va cho'zilishi mumkin; primerlarsiz, polimeraza bog'lanishi mumkin bo'lgan ikki zanjirli boshlanish joyi yo'q);[9] DNK maqsad mintaqasini to'ldiruvchi maxsus primerlar oldindan tanlanadi va ko'pincha laboratoriyada tayyorlanadi yoki tijorat biokimyoviy etkazib beruvchilardan sotib olinadi
- deoksinukleozid trifosfatlar, yoki dNTPlar (ba'zan "deoksinukleotid trifosfatlar" deb nomlanadi) nukleotidlar tarkibida trifosfat guruhlari), DNK polimeraza yangi DNK zanjirini sintez qiladigan qurilish bloklari
- a buferli eritma DNK polimerazasining optimal faolligi va barqarorligi uchun mos kimyoviy muhitni ta'minlash
- ikki valentli kationlar, odatda magniy (Mg) yoki marganets (Mn) ionlari; Mg2+ eng keng tarqalgan, ammo Mn2+ uchun ishlatilishi mumkin PCR vositasida DNK mutagenezi, yuqori Mn sifatida2+ konsentratsiya DNK sintezi paytida xato tezligini oshiradi;[10] va bir valentli kationlar, odatda kaliy (K) ionlari[yaxshiroq manba kerak ]
Reaksiya odatda 10-200 hajmda amalga oshiriladimk kichik reaksiya naychalarida (0,2-0,5 ml hajmda) a termal velosiped. Termal sikler reaktsiyaning har bir bosqichida talab qilinadigan haroratga erishish uchun reaksiya naychalarini isitadi va sovitadi (pastga qarang). Ko'pgina zamonaviy termal velosipedlar Peltier effekti bu PCR naychalarini ushlab turadigan blokni isitish va sovutish uchun elektr tokining teskari tomoniga o'tish orqali imkon beradi. Yupqa devorli reaktsiya naychalari qulaydir issiqlik o'tkazuvchanligi tez termal muvozanatni ta'minlash uchun. Ko'pgina termal tsiklchilar oldini olish uchun qizdirilgan qovoqlarga ega kondensatsiya reaktsiya naychasining yuqori qismida. Isitish qopqog'iga ega bo'lmagan eski termik velosipedlarga reaktsiya aralashmasi ustiga yog 'qatlami yoki kolba ichidagi mumi to'pi kerak.
Jarayon
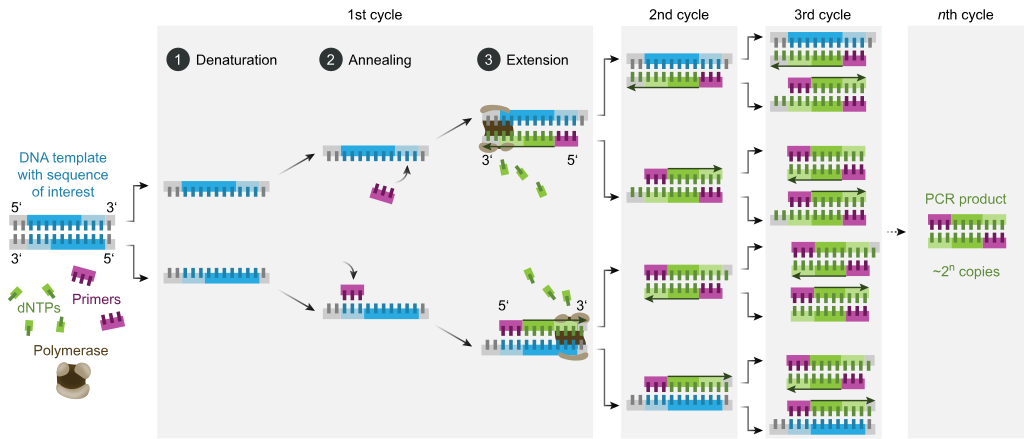
Odatda, PCR har xil tsikl odatda ikki yoki uchta alohida harorat bosqichlaridan iborat bo'lgan issiqlik tsikllari deb nomlangan 20-40 marta takrorlanadigan harorat o'zgarishidan iborat (quyidagi rasmga qarang). Velosipedda tez-tez juda yuqori haroratda (> 90 ° C (194 ° F)) bitta haroratli qadam qo'yiladi, so'ngra oxirgi mahsulotni uzaytirish yoki qisqa saqlash uchun oxirida bitta ushlab turiladi. Har bir tsikldagi ishlatilgan harorat va ularni qo'llash muddati turli xil parametrlarga, jumladan DNK sintezi uchun ishlatiladigan fermentga, reaktsiyadagi ikki valentli ionlar va dNTPlarning kontsentratsiyasiga va erish harorati (Tm) astarlardan.[11] Ko'pgina PCR usullari uchun odatiy qadamlar quyidagicha:
- Boshlash: Ushbu qadam faqat tomonidan issiqlik faolligini talab qiladigan DNK polimerazalari uchun talab qilinadi issiq boshlash PCR.[12] Bu reaktsiya kamerasini 94-96 ° C (201-205 ° F) haroratgacha yoki o'ta termostabil polimerazalar ishlatilsa, 98 ° C (208 ° F) qizdirishdan iborat bo'lib, keyin u 5-10 daqiqa ushlab turiladi.
- Denaturatsiya: Ushbu qadam velosiped harakatining birinchi muntazam hodisasi bo'lib, reaktsiya kamerasini 94–98 ° C (201–208 ° F) ga 20-30 soniya davomida qizdirishdan iborat. Bu sabab bo'ladi DNKning erishi, yoki ikki qatorli DNK shablonining sinishi natijasida denatürasyon vodorod aloqalari bir-biriga bog'langan ikkita DNK molekulasini hosil qiluvchi bir-birini to'ldiruvchi asoslar orasida.
- Tavlash: Keyingi bosqichda reaksiya harorati 20- 40 soniya davomida 50-65 ° C (122-149 ° F) ga tushirilib, primerlarni bitta zanjirli DNK shablonlarining har biriga yopishtirishga imkon beradi. Reaktsiya aralashmasiga odatda ikki xil primer kiradi: maqsadli mintaqani o'z ichiga olgan ikkita bir qatorli qo'shimchalarning har biri uchun bittasi. Astarlar o'zlari bir qatorli ketma-ketliklardir, lekin maqsad mintaqaning uzunligidan ancha qisqa, har bir ipning 3 ′ uchida faqat juda qisqa qatorlarni to'ldiradi.
- Tavlash pog'onasi uchun mos haroratni aniqlash juda muhim, chunki tavlanish harorati samaradorlik va o'ziga xoslikka kuchli ta'sir qiladi. Bunga imkon beradigan darajada past bo'lishi kerak duragaylash astarning ipga, lekin duragaylash o'ziga xos bo'lishi uchun etarlicha yuqori, ya'ni primer bog'lanishi kerak faqat ipning mukammal bir-birini to'ldiradigan qismiga va boshqa hech qanday joyga. Agar harorat juda past bo'lsa, primer mukammal darajada bog'lanishi mumkin. Agar u juda baland bo'lsa, astar umuman bog'lanmasligi mumkin. Odatda tavlanish harorati taxminan 3-5 ° S dan pastroq Tm ishlatilgan astarlardan. Bir-birini to'ldiruvchi asoslar orasidagi barqaror vodorod aloqalari faqat primer ketma-ketligi shablon ketma-ketligiga juda mos kelganda hosil bo'ladi. Ushbu bosqichda polimeraza primer-shablon gibridiga bog'lanib, DNK hosil bo'lishini boshlaydi.
- Uzaytirish / cho'zish: Ushbu bosqichdagi harorat ishlatilgan DNK polimeraziga bog'liq; tegmaslik faoliyat ning termostabil DNK polimerazasi uchun harorat Taq polimeraza taxminan 75-80 ° C (167-176 ° F),[13][14] bu ferment bilan odatda 72 ° C (162 ° F) harorat ishlatiladi. Ushbu bosqichda DNK polimeraza reaksiya aralashmasidan erkin dNTPlarni qo'shib DNK shablon zanjiriga qo'shimcha bo'lgan yangi DNK zanjirini sintez qiladi. 5′ dan 3 ′ gacha yo'nalish, kondensatsiya 5′-fosfat guruhi dNTPlarning 3′-gidroksi guruhi paydo bo'lgan (cho'ziluvchan) DNK zanjiri oxirida. Uzayish uchun zarur bo'lgan aniq vaqt, ishlatilgan DNK polimeraziga va kuchayish uchun DNK maqsad mintaqasining uzunligiga bog'liq. Odatda DNK polimerazalari eng maqbul haroratda daqiqada ming asosni polimerlashadi. Optimal sharoitlarda (ya'ni, cheklovchi substratlar yoki reagentlar tufayli cheklovlar bo'lmasa), har bir kengayish / cho'zish bosqichida DNK nishonlari ketma-ketligi soni ikki baravar oshiriladi. Har bir ketma-ket tsikl bilan original shablon iplari va barcha yangi hosil qilingan iplar keyingi cho'zilish davri uchun shablon zanjiriga aylanadi va bu aniq DNK maqsad mintaqasini eksponent (geometrik) kuchayishiga olib keladi.
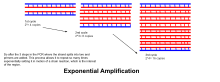
- Denaturatsiya, tavlanish va cho'zish jarayonlari yagona tsiklni tashkil etadi. DNK nishonini millionlab nusxalarda kuchaytirish uchun bir nechta tsikllar talab qilinadi. Belgilangan sikllar sonidan keyin hosil bo'lgan DNK nusxalari sonini hisoblash uchun ishlatiladigan formula 2 ga tengn, qayerda n tsikllar soni. Shunday qilib, 30 tsikl uchun o'rnatilgan reaktsiya 2 ga olib keladi30yoki 1,073,741,824 nusxadagi asl DNK maqsad mintaqasining nusxasi.
- Oxirgi cho'zish: Ushbu bitta qadam ixtiyoriy, ammo oxirgi PCRdan keyin 5-15 daqiqa davomida 70-74 ° C (PCRda ishlatiladigan ko'p polimerazalarning optimal faolligi uchun zarur bo'lgan harorat oralig'ida) haroratda bajariladi. Qolgan bitta zanjirli DNKning to'liq cho'zilishini ta'minlash uchun tsikl.
- Yakuniy ushlab turish: Oxirgi qadam reaktsiya kamerasini 4-15 ° C (39-59 ° F) ga qadar noma'lum vaqtgacha sovutadi va PCR mahsulotlarini qisqa muddatli saqlash uchun ishlatilishi mumkin.

PCR kutilgan DNK nishon mintaqasini muvaffaqiyatli ishlab chiqarganligini tekshirish uchun (ba'zida uni kuchaytiruvchi yoki deyiladi) amplikon ), agarozli gel elektroforez PCR mahsulotlarini hajmini ajratish uchun ishlatilishi mumkin. PCR mahsulotlarining hajmi a bilan taqqoslash orqali aniqlanadi DNK narvon, ma'lum miqdordagi DNK parchalarini o'z ichiga olgan molekulyar og'irlik markeri, u PCR mahsulotlari bilan bir qatorda gelda ishlaydi.

Bosqichlar
Boshqa kimyoviy reaktsiyalar singari, PCR ning reaktsiya tezligi va samaradorligiga cheklovchi omillar ta'sir qiladi. Shunday qilib, butun PCR jarayonini reaktsiya rivojlanishiga qarab uch bosqichga bo'lish mumkin:
- Eksponentli amplifikatsiya: Har bir tsiklda mahsulot miqdori ikki baravar ko'paytiriladi (100% reaktsiya samaradorligini nazarda tutgan holda). 30 tsikldan so'ng DNKning bitta nusxasini 1 000 000 000 (bir milliard) nusxaga etkazish mumkin. Demak, ma'lum bir ma'noda, DNKning alohida zanjirining replikatsiyasi nazorat ostida bo'lgan sharoitda naychada boshqariladi.[15] Reaksiya juda sezgir: faqat DNKning bir necha daqiqali miqdori bo'lishi kerak.
- Sahnadan chiqib ketish: DNK polimeraza faolligini yo'qotganda va dNTPlar va primerlar kabi reaktivlarni iste'mol qilish ularning cheklanishiga olib kelishi bilan reaktsiya sekinlashadi.
- Plato: Reaktivlar va fermentlarning charchashidan boshqa mahsulot to'planib qolmaydi.
Optimallashtirish
Amalda, PCR turli sabablarga ko'ra ishlamay qolishi mumkin, bu qisman ifloslanishga sezgirligi tufayli soxta DNK mahsulotlarini ko'payishiga olib keladi. Shu sababli, PCR sharoitlarini optimallashtirish bo'yicha bir qator texnik va protseduralar ishlab chiqilgan.[16][17] Tashqi DNK bilan ifloslanish laboratoriya protokollari va PCRgacha bo'lgan aralashmalarni potentsial DNK ifloslantiruvchi moddalardan ajratib turadigan protseduralar bilan hal qilinadi.[7] Bu, odatda, PCR mahsulotlarini tahlil qilish yoki tozalash uchun maydonlardan, bir martalik ishlatiladigan plastmassadan foydalanish va reaksiya sozlamalari orasidagi ish joyini yaxshilab tozalashdan PCR-o'rnatish maydonlarini fazoviy ajratishni o'z ichiga oladi. Astarni loyihalash texnikasi PCR mahsulotining unumdorligini oshirishda va soxta mahsulotlarning paydo bo'lishining oldini olishda muhim ahamiyatga ega va alternativ bufer komponentlari yoki polimeraza fermentlarini ishlatish DNKning uzoq yoki boshqa muammoli hududlarini kuchaytirishga yordam beradi. Kabi reaktivlarni qo'shish formamid, bufer tizimlarida PCR ning o'ziga xosligi va rentabelligini oshirishi mumkin.[18] PCR nazariy natijalarini kompyuter simulyatsiyasi (Elektron PCR ) primer dizaynida yordam berish uchun bajarilishi mumkin.[19]
Ilovalar
Tanlangan DNK izolatsiyasi
PCR, DNKning ma'lum bir mintaqasini selektiv ravishda kuchaytirish orqali genomik DNKdan DNK fragmentlarini ajratishga imkon beradi. PCR-dan foydalanish ishlab chiqarish kabi ko'plab usullarni kuchaytiradi duragaylash zondlari uchun Janubiy yoki shimoliy duragaylash va DNKni klonlash, bu ma'lum bir DNK mintaqasini ifodalovchi ko'proq DNK miqdorini talab qiladi. PCR ushbu texnikani yuqori miqdordagi sof DNK bilan ta'minlaydi, bu juda oz miqdordagi boshlang'ich materialdan ham DNK namunalarini tahlil qilishga imkon beradi.
PCR ning boshqa dasturlariga quyidagilar kiradi DNKning ketma-ketligi kuchaytiruvchi primerlardan biri ishlatilishi mumkin bo'lgan noma'lum PCR-kuchaytirilgan ketma-ketliklarini aniqlash Sanger ketma-ketligi, DNK ketma-ketligini kiritishni o'z ichiga olgan rekombinant DNK texnologiyalarini tezlashtirish uchun DNK ketma-ketligini ajratish plazmid, fag, yoki kosmid (o'lchamiga qarab) yoki boshqa organizmning genetik materiallari. Bakterial koloniyalar (kabi E. coli ) to'g'ri DNK uchun PCR yordamida tezda tekshirilishi mumkin vektor konstruktsiyalar.[20] PCR uchun ham foydalanish mumkin genetik barmoq izlari; turli xil PCR asosidagi usullar orqali eksperimental DNKlarni taqqoslash orqali odam yoki organizmni aniqlash uchun ishlatiladigan sud texnikasi.

- Ota
- Bola
- Ona
Bola ota-onasining har birining barmoq izlarining hammasiga ham bir nechtasini meros qilib olgan, bu yangi, noyob barmoq izini bergan.
Ba'zi PCR barmoq izlari usullari yuqori diskriminatsion kuchga ega va ota-bola yoki aka-ukalar singari shaxslar o'rtasidagi genetik munosabatlarni aniqlash uchun ishlatilishi mumkin va otalikni tekshirishda qo'llaniladi (4-rasm). Ushbu uslub ma'lum molekulyar soatlar ishlatilganda organizmlar o'rtasidagi evolyutsion munosabatlarni aniqlash uchun ham ishlatilishi mumkin (ya'ni 16S rRNK va mikroorganizmlarning recA genlari).[21]
DNKni kuchaytirish va miqdorini aniqlash
PCR DNKni maqsad qilgan mintaqalarini kuchaytirgani uchun PCR ni juda oz miqdordagi namunalarni tahlil qilish uchun ishlatish mumkin. Bu ko'pincha juda muhimdir sud ekspertizasi, dalil sifatida faqat DNKning iz miqdori mavjud bo'lganda. PCR tahlil qilishda ham qo'llanilishi mumkin qadimiy DNK bu o'n ming yillardir. Ushbu PCR asosidagi usullar, masalan, qirq ming yillik hayvonlarda muvaffaqiyatli qo'llanilgan mamont Misrni tahlil qilishgacha bo'lgan dasturlarda, shuningdek inson DNKida mumiyalar a identifikatsiyasiga Ruscha podshoh va ingliz qiroli tanasi Richard III.[22]
Miqdor PCR yoki real vaqtda PCR (qPCR,[23] bilan aralashmaslik kerak RT-PCR ) usullar namunadagi mavjud ketma-ketlik miqdorini baholashga imkon beradi - bu darajalarni miqdoriy aniqlash uchun ko'pincha qo'llaniladigan usul gen ekspressioni. Miqdoriy PCR har bir PCR amplifikatsiyasidan so'ng DNK mahsulotining to'planishini o'lchaydigan DNK miqdorini aniqlash uchun yaratilgan vosita.
qPCR sintez jarayoni sodir bo'lganda kontsentratsiyani o'lchaganligi sababli real vaqtda DNKning aniq ketma-ketligini miqdoriy aniqlash va aniqlashga imkon beradi. Bir vaqtning o'zida aniqlash va miqdorini aniqlashning ikkita usuli mavjud. Birinchi usul foydalanishdan iborat lyuminestsent qo'shaloq iplar orasida o'ziga xos bo'lmagan holda saqlanadigan bo'yoqlar. Ikkinchi usul ma'lum ketma-ketliklar uchun kod yozadigan va lyuminestsent yorliqli problarni o'z ichiga oladi. Ushbu usullar yordamida DNKni aniqlashni faqat uning qo'shimcha DNK bilan zondlarni duragaylash jarayoni sodir bo'lgandan keyingina ko'rish mumkin. Qiziqarli texnik kombinatsiya - bu real vaqtda PCR va teskari transkriptsiya. RT-qPCR deb nomlangan ushbu murakkab texnika oz miqdordagi RNK miqdorini aniqlashga imkon beradi. Ushbu estrodiol texnika orqali mRNA cDNA ga aylanadi, bu esa qPCR yordamida qo'shimcha ravishda aniqlanadi. Ushbu usul PCR ning so'nggi nuqtasida xatolik ehtimolini pasaytiradi,[24] saraton kabi genetik kasalliklar bilan bog'liq genlarni aniqlash imkoniyatini oshirish.[4] Laboratoriyalarda RT-qPCR dan genlarni regulyatsiyasini sezgir o'lchash maqsadida foydalaniladi. PCR ning ishonchli miqdorini aniqlashning matematik asoslari[25] va RT-qPCR[26] tadqiqot, tibbiy, diagnostika va yuqumli kasalliklar qo'llanilishida eksperimental ma'lumotlarning aniq joylashtirilgan tartiblarini amalga oshirishni osonlashtirish.[27][28][29][30]
Tibbiy va diagnostik qo'llanmalar
Kelajakdagi ota-onalarning mavjudligini tekshirish mumkin genetik tashuvchilar yoki ularning farzandlari a ta'sir qilganligi uchun sinovdan o'tishlari mumkin kasallik.[1] Uchun DNK namunalari prenatal test tomonidan olinishi mumkin amniyosentez, chorionik villusdan namuna olish, yoki hatto onaning qonida aylanadigan noyob xomilalik hujayralarni tahlil qilish orqali. PCRni tahlil qilish ham juda muhimdir preimplantatsiya genetik diagnostikasi, bu erda rivojlanayotgan embrionning alohida hujayralari mutatsiyalar uchun sinovdan o'tkaziladi.
- PCR shuningdek sezgir sinovning bir qismi sifatida ishlatilishi mumkin to'qimalarni terish, hayotiy organ transplantatsiyasi. 2008 yildan boshlab,[yangilash] uchun antikorlarga asoslangan an'anaviy testlarni almashtirish taklifi ham bor qon guruhi PCR asosidagi testlar bilan.[31]
- Saratonning ko'plab shakllari o'zgarishni o'z ichiga oladi onkogenlar. Ushbu mutatsiyalarni o'rganish uchun PCR asosidagi testlardan foydalangan holda, ba'zida terapiya sxemalari bemorga individual ravishda moslashtirilishi mumkin. PCR erta tashxis qo'yishga imkon beradi zararli kabi kasalliklar leykemiya va limfomalar, hozirgi kunda saraton kasalligini o'rganish bo'yicha eng yuqori darajada rivojlangan va allaqachon muntazam ravishda ishlatilib kelinmoqda. PCR tahlillari to'g'ridan-to'g'ri genomik DNK namunalarida translokatsiyaga xos malign hujayralarni boshqa usullarga qaraganda kamida 10 000 baravar yuqori sezgirlikda aniqlash uchun amalga oshirilishi mumkin.[32] PCR tibbiy sohada juda foydalidir, chunki u o'simta supressorlarini ajratish va kuchaytirishga imkon beradi. Masalan, miqdoriy PCR yordamida bitta hujayralarni miqdorini aniqlash va tahlil qilish, shuningdek DNK, mRNK va oqsillarni tasdiqlash va birikmalarini tanib olish mumkin.[24]
Yuqumli kasalliklarga qarshi dasturlar
PCR yuqumli kasalliklarni, shu jumladan bakteriyalar yoki viruslar sabab bo'lgan kasalliklarni tez va yuqori darajada aniq tashxislash imkonini beradi.[33] PCR shuningdek, etishtirilmaydigan yoki sekin o'sadigan mikroorganizmlarni aniqlashga ruxsat beradi mikobakteriyalar, anaerob bakteriyalar, yoki viruslar dan to'qima madaniyati tahlillar va hayvon modellari. Mikrobiologiyada PCR diagnostikasi uchun asos - bu yuqumli kasalliklarni aniqlash va patogen bo'lmagan shtammlardan patogen bo'lmaganlarni ma'lum genlar asosida ajratish.[33][34]
Yuqumli kasalliklarga chalingan organizmlarni tavsiflash va aniqlash quyidagi usullarda amalga oshirildi:
- The inson immunitet tanqisligi virusi (yoki OIV ), topish va yo'q qilish qiyin maqsaddir. INFEKTSION uchun dastlabki sinovlar qonda aylanadigan virusga qarshi antikorlarning mavjudligiga bog'liq edi. Ammo antikorlar infektsiyadan ko'p hafta o'tgach paydo bo'lmaydi, onalik antikorlari yangi tug'ilgan chaqaloq infektsiyasini yashiradi va infektsiyaga qarshi kurashadigan terapevtik vositalar antikorlarga ta'sir qilmaydi. PCR testlar 50 000 dan ortiq xujayra hujayralarining DNKlari orasida bitta virusli genomni aniqlay oladigan ishlab chiqilgan.[35] Infektsiyalarni ilgari aniqlash mumkin, donorlik qonini to'g'ridan-to'g'ri virusga tekshirib ko'rish, yangi tug'ilgan chaqaloqlarni zudlik bilan yuqtirish uchun sinovdan o'tkazish va virusga qarshi davolanishning ta'siri miqdoriy.
- Ba'zi kasallik organizmlari, masalan sil kasalligi, bemorlardan namuna olish qiyin va sekin olinadi o'sgan laboratoriyada. PCR asosida o'tkazilgan testlar ozgina miqdorda kasallik organizmlarini (tirik yoki o'lik) qulay sharoitda aniqlashga imkon berdi namunalar. Batafsil genetik tahlil antibiotiklarga chidamliligini aniqlash uchun ham qo'llanilishi mumkin, bu darhol va samarali terapiyani amalga oshirishga imkon beradi. Terapiya ta'sirini ham darhol baholash mumkin.
- A ning tarqalishi kasallik organizm populyatsiyalari orqali ichki yoki yovvoyi hayvonlar PCR sinovlari orqali kuzatilishi mumkin. Ko'p hollarda yangi virusli kasallik paydo bo'lishi kichik turlari aniqlanishi va kuzatilishi mumkin. Mas'ul bo'lgan organizmning kichik turlari oldingi epidemiyalar shuningdek, PCR tahlillari bilan aniqlanishi mumkin.
- Virusli DNKni PCR yordamida aniqlash mumkin. Amaldagi primerlar virus DNKsidagi maqsadli ketma-ketliklarga xos bo'lishi kerak va PCR virusli genomning diagnostik tahlillari yoki DNK sekvensiyasi uchun ishlatilishi mumkin. PCR ning yuqori sezuvchanligi virusni yuqtirishdan ko'p o'tmay va hatto kasallik boshlanishidan oldin aniqlashga imkon beradi.[33] Bunday erta aniqlash shifokorlarga davolanishda muhim vaqtni berishi mumkin. Virus miqdori ("virusli yuk ") bemorda PCR asosida DNK miqdorini aniqlash texnikasi bilan ham aniqlanishi mumkin (pastga qarang).RT-PCR ) DNKni emas, balki virusli RNKni aniqlash uchun ishlatiladi: bu testda teskari transkriptaz fermenti virusli RNK bilan mos keladigan DNK ketma-ketligini yaratish uchun ishlatiladi; keyinchalik bu DNK odatdagi PCR usuli bo'yicha kuchaytiriladi. RT-PCR SARS-CoV-2 virusli genomini aniqlash uchun keng qo'llaniladi.[36]
- Ko'k yo'tal kabi kasalliklar (yoki ko'k yo'tal ) bakteriyalar tomonidan kelib chiqadi Bordetella yo'tal. Ushbu bakteriyalar turli xil hayvonlar va odamlarga ta'sir qiladigan va ko'plab yosh bolalarning o'limiga olib keladigan jiddiy o'tkir respiratorli infektsiya bilan ajralib turadi. Ko'k yo'tal toksini - bu hujayra retseptorlari bilan ikki o'lchov bilan bog'lanib, hujayralar immunitetida rol o'ynaydigan T limfotsitlar kabi har xil hujayra turlari bilan reaksiyaga kirishadigan ekzotoksin oqsilidir.[37] PCR - bu ko'kyo'tal toksini uchun gen ichidagi ketma-ketlikni aniqlaydigan muhim sinov vositasi. PCR toksin uchun yuqori sezuvchanlikka va tez aylanish vaqtiga ega bo'lganligi sababli, kulturaga nisbatan ko'kyo'talni tashxislash uchun juda samarali.[38]
Sud ekspertizasi arizalari
PCR asosida ishlab chiqish genetik (yoki DNK ) barmoq izlari protokollari keng qo'llanilishini ko'rdi sud tibbiyoti:
- Eng kamsituvchi shaklida, genetik barmoq izlari har qanday kishini butun aholidan noyob tarzda ajratishi mumkin dunyo. DNKning daqiqali namunalarini a dan ajratish mumkin jinoyat joyi va taqqoslangan gumon qilinuvchilardan yoki a DNK ma'lumotlar bazasi oldingi dalillar yoki mahkumlarning. Ushbu testlarning sodda versiyalari ko'pincha jinoiy tergov paytida gumon qilinuvchilarni tezda chiqarib tashlash uchun ishlatiladi. Bir necha o'n yillik jinoyatlarning dalillari tekshirilishi mumkin, tasdiqlovchi yoki oqlovchi dastlab sudlangan odamlar.
- Sud-tibbiy DNKni terish jinoyat sodir etilgan joyda topilgan dalillarni tahlil qilish natijasida jinoiy gumon qilinuvchilarni aniqlash yoki oqlashning samarali usuli bo'ldi. Inson genomida ko'plab takrorlanadigan mintaqalar mavjud, ular genlar ketma-ketligida yoki genomning kodlanmagan hududlarida uchraydi. Xususan, inson DNKning 40% gacha takrorlanadi.[4] Genomda takrorlanadigan, kodlanmaydigan mintaqalar uchun ikkita alohida toifalar mavjud. Birinchi toifaga o'zgaruvchan sonli tandem takrorlanishi (VNTR) deyiladi, ularning uzunligi 10-100 tayanch jufti, ikkinchi toifasi esa qisqa tandem takrorlanishi (STR) deb nomlanadi va ular takrorlanadigan 2-10 tayanch juftlik qismlaridan iborat. PCR bir necha taniqli VNTR va STRlarni takrorlanadigan har bir mintaqaning yon tomonida joylashgan primerlar yordamida kuchaytirish uchun ishlatiladi. Har bir STR uchun har qanday shaxsdan olingan bo'laklarning o'lchamlari qaysi allellar mavjudligini ko'rsatadi. Shaxs uchun bir nechta STRni tahlil qilib, har bir kishi uchun allellar to'plami statistik jihatdan noyob bo'lishi mumkinligi aniqlanadi.[4] Tadqiqotchilar inson genomining to'liq ketma-ketligini aniqladilar. Ushbu ketma-ketlikka NCBI veb-sayti orqali osongina kirish mumkin va ko'plab hayotiy dasturlarda qo'llaniladi. Masalan, Federal qidiruv byurosi identifikatsiya qilish uchun foydalaniladigan DNK markerlari to'plamini tuzdi va ular DNKning birlashgan indekslari tizimi (CODIS) DNK ma'lumotlar bazasi deb ataladi.[4] Ushbu ma'lumotlar bazasidan foydalanish DNK namunasining mos kelish ehtimolligini aniqlash uchun statistik tahlillardan foydalanishga imkon beradi. PCR sud-tibbiy DNKni terish uchun ishlatiladigan juda kuchli va muhim analitik vositadir, chunki tadqiqotchilarga tahlil qilish uchun maqsadli DNKning juda oz miqdori kerak. Masalan, soch follikulasi biriktirilgan bitta odamning sochlarida tahlilni o'tkazish uchun etarli DNK mavjud. Xuddi shunday, bir nechta sperma, tirnoq ostidagi teri namunalari yoki oz miqdordagi qon yakuniy tahlil uchun etarli DNKni ta'minlay oladi.[4]
- Kamroq kamsituvchi shakllari DNK barmoq izlari yordam berishi mumkin DNKning otalikni tekshirishi, bu erda individual yaqin qarindoshlari bilan mos keladi. Odamlarning noma'lum qoldiqlaridan olingan DNKni sinab ko'rish mumkin va ularni ota-onalari, aka-ukalari yoki farzandlari bilan taqqoslash mumkin. Shunga o'xshash test asrab olingan (yoki o'g'irlangan) bolaning biologik ota-onasini tasdiqlash uchun ishlatilishi mumkin. Yangi tug'ilgan chaqaloqning haqiqiy biologik otasi ham bo'lishi mumkin tasdiqlangan (yoki chiqarib tashlangan).
- PCR AMGX / AMGY dizayni nafaqat ko'rsatildi[tushuntirish kerak ] juda oz miqdordagi genomdan DNK sekanslarini kuchaytirishga yordam beradi. Shu bilan birga, sud-tibbiyot suyagi namunalaridan real vaqtda jinsiy aloqani aniqlash uchun ham foydalanish mumkin. Bu sud-tergov ishlari va qadimiy namunalarda jinsni aniqlashning kuchli va samarali usulini taqdim etadi.[39]
Tadqiqot dasturlari
PCR molekulyar genetika bo'yicha ko'plab tadqiqot sohalarida qo'llanilgan:
- PCR, DNKning qisqa parchalarini tezkor ravishda ishlab chiqarishga imkon beradi, hattoki ikkita primerning ketma-ketligi ma'lum bo'lsa ham. PCR ning bu qobiliyati ishlab chiqarish kabi ko'plab usullarni kuchaytiradi duragaylash zondlar uchun Janubiy yoki shimoliy blot duragaylash. PCR ushbu texnikani juda ko'p miqdordagi sof DNK bilan ta'minlaydi, ba'zan esa bitta ip bo'lib, juda oz miqdordagi boshlang'ich materiallardan ham tahlil qilishga imkon beradi.
- Vazifasi DNKning ketma-ketligi PCR ham yordam berishi mumkin. DNKning ma'lum segmentlari genetik kasallik mutatsiyasiga ega bo'lgan bemordan osonlikcha hosil bo'lishi mumkin. Amplifikatsiya texnikasining modifikatsiyalari umuman noma'lum genomdan segmentlarni ajratib olishi yoki qiziqish doirasining faqat bitta yo'nalishini yaratishi mumkin.
- PCR ning an'anaviy jarayoniga oid ko'plab dasturlar mavjud DNKni klonlash. U kattaroq genomdan vektorga kiritish uchun segmentlarni ajratib olishi mumkin, bu faqat oz miqdorda bo'lishi mumkin. Bitta "vektorli primer" to'plamidan foydalanib, u allaqachon vektorlarga kiritilgan qismlarni tahlil qilishi yoki ajratib olishi mumkin. PCR protokoliga ba'zi o'zgartirishlar kiritilishi mumkin mutatsiyalar hosil qilish kiritilgan qismning (umumiy yoki saytga yo'naltirilgan).
- Ketma-ketlik bilan belgilangan saytlar PCR genomning ma'lum bir segmenti ma'lum bir klonda mavjudligini ko'rsatuvchi ko'rsatkich sifatida ishlatiladigan jarayondir. The Inson genomining loyihasi ushbu dasturni ular ketma-ketlikdagi kosmid klonlarini xaritalash va turli laboratoriyalar natijalarini muvofiqlashtirish uchun muhim deb topdi.
- PCR dasturi bu filogenik dan DNKni tahlil qilish qadimiy manbalar, masalan, tiklangan suyaklarda topilgan Neandertallar, ning muzlatilgan to'qimalaridan mamontlar yoki Misr mumiyalarining miyasidan.[15] Ba'zi hollarda ushbu manbalardan yuqori darajada parchalanib ketgan DNK kuchaytirilishning dastlabki bosqichida qayta to'planishi mumkin.
- PCR ning keng tarqalgan qo'llanilishi bu naqshlarni o'rganishdir gen ekspressioni. To'qimalarni (yoki hatto alohida hujayralarni) har xil bosqichlarda tahlil qilib, qaysi genlar faollashganini yoki o'chirilganligini ko'rish mumkin. Ushbu dastur ham foydalanishi mumkin miqdoriy PCR ifoda etishning haqiqiy darajalarini aniqlash
- PCR qobiliyati bir vaqtning o'zida individual spermatozoidlardan bir nechta lokuslarni kuchaytirish[40] ning an’anaviy vazifasini ancha oshirdi genetik xaritalash o'qish orqali xromosoma kesishmalari keyin mayoz. Juda yaqin lokuslar orasidagi nodir krossover hodisalari to'g'ridan-to'g'ri minglab individual spermatozoidlarni tahlil qilish orqali kuzatilgan. Xuddi shunday, odatiy bo'lmagan o'chirishlar, qo'shimchalar, translokatsiyalar yoki inversiyalarni ham urug'lantirish, embriogenez va hokazo uzoq va mashaqqatli jarayonlarni kutish (yoki to'lash) shart bo'lmasdan tahlil qilish mumkin.
- Saytga yo'naltirilgan mutagenez: PCR yordamida olimlar o'z ixtiyori bilan tanlagan mutatsiyalar bilan mutant genlarni yaratish uchun foydalanish mumkin. Ushbu mutatsiyalarni oqsillar o'z funktsiyalarini qanday bajarishini tushunish va oqsil funktsiyasini o'zgartirish yoki yaxshilash uchun tanlash mumkin.
Afzalliklari
PCR bir qator afzalliklarga ega. Tushunish va ishlatish juda sodda va natijalarni tezda keltirib chiqaradi. Texnika ketma-ketlik, klonlash va tahlil qilish uchun ma'lum bir mahsulotning milliondan milliardlab nusxalarini ishlab chiqarish qobiliyatiga ega bo'lganligi bilan juda sezgir. qRT-PCR sintez qilingan mahsulot miqdorini aniqlashning qo'shimcha afzalligi bilan PCR bilan bir xil afzalliklarga ega. Shuning uchun u o'smalar, mikroblar yoki boshqa kasallik holatlarida gen ekspression darajalarining o'zgarishini tahlil qilish uchun foydalanadi.[24]
PCR juda kuchli va amaliy tadqiqot vositasidir. Ko'pgina kasalliklarning noma'lum etiologiyalarini ketma-ketligini PCR aniqlaydi. Ushbu uslub ilgari ma'lum bo'lgan viruslar bilan bog'liq bo'lgan ilgari noma'lum viruslar ketma-ketligini aniqlashga yordam beradi va shu bilan kasallikning o'zi haqida yaxshiroq ma'lumot beradi. Agar protsedurani yanada soddalashtirish mumkin bo'lsa va sezgir radiometrik bo'lmagan tizimlarni yaratish mumkin bo'lsa, PCR kelgusi yillar davomida klinik laboratoriyada taniqli o'rinni egallaydi.[15]
Cheklovlar
PCR-ning asosiy cheklovlaridan biri shundaki, maqsadli ketma-ketlik to'g'risida oldindan ma'lumot uning tanlab kuchaytirilishiga imkon beradigan primerlarni yaratish uchun zarurdir.[24] Bu shuni anglatadiki, odatda, PCR foydalanuvchilari DNK polimerazining primer-shablon duragaylari bilan to'g'ri bog'lanishini va keyinchalik hosil bo'lishini ta'minlash uchun ikkita bitta ipli shablonning har birida maqsadli mintaqaning yuqori qismida aniq ketma-ketlikni bilishlari kerak. DNK sintezi paytida butun maqsadli mintaqa.
Barcha fermentlar singari, DNK polimerazalari ham xatolarga moyil bo'lib, bu o'z navbatida hosil bo'lgan PCR fragmentlarida mutatsiyalarni keltirib chiqaradi.[41]
PCR-ning yana bir cheklovi shundaki, hatto eng kichik miqdordagi ifloslantiruvchi DNK kuchaytirilishi mumkin, natijada noto'g'ri yoki noaniq natijalarga olib keladi. Ifloslanish ehtimolini minimallashtirish uchun tergovchilar reagent tayyorlash, PCR va mahsulotni tahlil qilish uchun alohida xonalarni zaxiralashlari kerak. Reaktivlar bir martalik ishlatilishi kerak bitiklar. Bir martali ishlatiladigan pistonli pipetkalar va qo'shimcha uzun pipetka uchlari muntazam ravishda ishlatilishi kerak.[15]
Hum kislotalarini o'z ichiga olgan atrof-muhit namunalari PCR kuchayishini inhibe qilishi va noto'g'ri natijalarga olib kelishi mumkin.
O'zgarishlar
- Allelga xos PCR: bitta nukleotidli o'zgarishlarga asoslangan diagnostika yoki klonlash texnikasi (SNVlar bilan aralashmaslik kerak SNPlar ) (bemorda bitta asosli farqlar). Buning uchun DNK ketma-ketligi, shu bilan orasidagi farqlarni oldindan bilish kerak allellar, va 3 'uchlari SNVni qamrab oladigan primerlardan foydalanadi (odatda SNV atrofidagi asosiy juftlik buferi). Qattiq sharoitlarda PCRni kuchaytirish shablon va primer o'rtasida nomuvofiqlik mavjud bo'lganda ancha kam samaralidir, shuning uchun SNPga xos bo'lgan primer bilan muvaffaqiyatli amplifikatsiya ketma-ketlikda ma'lum SNP mavjudligini signal qiladi.[42] Qarang SNP genotipini yaratish qo'shimcha ma'lumot olish uchun.
- PCR yig'ish yoki Polimeraza velosiped assambleyasi (PCA): qisqa segmentlar bilan uzun oligonukleotidlar havzasida PCR o'tkazib, uzoq DNK sekanslarini sun'iy sintezi. Oligonukleotidlar sezgi va antisens yo'nalishlarini almashtiradi va bir-birining ustiga tushgan segmentlar PCR parchalarining tartibini belgilaydi va shu bilan tanlab yakuniy uzun DNK mahsulotini ishlab chiqaradi.[43]
- Asimmetrik PCR: ikki qatorli DNK shablonidagi bitta DNK zanjirini imtiyozli ravishda kuchaytiradi. Bu ishlatiladi ketma-ketlik va ikkita qo'shimcha chiziqlardan faqat bittasini kuchaytirish zarur bo'lgan gibridizatsiya tekshiruvi. PCR odatdagidek amalga oshiriladi, ammo kuchaytirishga mo'ljallangan ip uchun juda ko'p miqdordagi primer bilan. Sekin bo'lgani uchun (arifmetik ) cheklovchi primer ishlatilgandan keyin reaktsiyada kuchayish, PCR ning qo'shimcha tsikllari talab qilinadi.[44] Sifatida tanilgan ushbu jarayonga yaqinda kiritilgan o'zgartirish Linear-AuzoqTu-Exponential-PCR (LATE-PCR), eritish harorati yuqori bo'lgan cheklovchi primerdan foydalanadi (Tm ) reaktsiyaning samaradorligini saqlab qolish uchun ortiqcha primerga qaraganda cheklangan primer kontsentratsiyasi o'rta reaktsiyani pasaytiradi.[45]
- Konvektiv PCR: PCRni bajarishning psevdoizotermik usuli. PCR aralashmasini qayta-qayta isitish va sovutish o'rniga, eritma termal gradiyentga ta'sir qiladi. Natijada paydo bo'lgan termal beqarorlik konvektiv oqimi PCR reagentlarini issiq va sovuq mintaqalardan avtomatik ravishda aralashtirib, PCRni qayta-qayta ta'minlaydi.[46] PCR korpusining termal chegara shartlari va geometriyasi kabi parametrlar xaotik oqim maydonlarining paydo bo'lishidan foydalanish orqali mustahkam va tezkor PCR hosil qilish uchun optimallashtirilishi mumkin.[47] Bunday konvektiv oqim PCRni sozlash qurilmaning quvvat talabini va ishlash vaqtini sezilarli darajada qisqartiradi.
- Dial-PCR: gen sintezi uchun aniq DNK molekulalarini olish uchun juda parallel usul. A complex library of DNA molecules is modified with unique flanking tags before massively parallel sequencing. Tag-directed primers then enable the retrieval of molecules with desired sequences by PCR.[48]
- Raqamli PCR (dPCR): used to measure the quantity of a target DNA sequence in a DNA sample. The DNA sample is highly diluted so that after running many PCRs in parallel, some of them do not receive a single molecule of the target DNA. The target DNA concentration is calculated using the proportion of negative outcomes. Hence the name 'digital PCR'.
- Helicazga bog'liq amplifikatsiya: similar to traditional PCR, but uses a constant temperature rather than cycling through denaturation and annealing/extension cycles. DNK-helikaza, an enzyme that unwinds DNA, is used in place of thermal denaturation.[49]
- PCR-ni issiq boshlash: a technique that reduces non-specific amplification during the initial set up stages of the PCR. It may be performed manually by heating the reaction components to the denaturation temperature (e.g., 95 °C) before adding the polymerase.[50] Specialized enzyme systems have been developed that inhibit the polymerase's activity at ambient temperature, either by the binding of an antikor[12][51] or by the presence of covalently bound inhibitors that dissociate only after a high-temperature activation step. Hot-start/cold-finish PCR is achieved with new hybrid polymerases that are inactive at ambient temperature and are instantly activated at elongation temperature.
- Silikon PCRda (digital PCR, virtual PCR, electronic PCR, e-PCR) refers to computational tools used to calculate theoretical polymerase chain reaction results using a given set of astarlar (zondlar ) kuchaytirish uchun DNK ketma-ketlikdan ketma-ketliklar genom yoki transkriptom. In silico PCR was proposed as an educational tool for molecular biology.[52]
- Intersequence-specific PCR (ISSR): a PCR method for DNA fingerprinting that amplifies regions between simple sequence repeats to produce a unique fingerprint of amplified fragment lengths.[53]
- Teskari PCR: is commonly used to identify the flanking sequences around genomik inserts. Bu bir qatorni o'z ichiga oladi DNA digestions va self ligation, resulting in known sequences at either end of the unknown sequence.[54]
- Ligation-mediated PCR: uses small DNA linkers ligated to the DNA of interest and multiple primers annealing to the DNA linkers; it has been used for DNKning ketma-ketligi, genome walking va DNK izlari.[55]
- Metilatsiyaga xos PCR (MSP): developed by Stephen Baylin va Jeyms G. Xerman at the Johns Hopkins School of Medicine,[56] and is used to detect methylation of CpG islands in genomic DNA. DNA is first treated with sodium bisulfite, which converts unmethylated cytosine bases to uracil, which is recognized by PCR primers as thymine. Two PCRs are then carried out on the modified DNA, using primer sets identical except at any CpG islands within the primer sequences. At these points, one primer set recognizes DNA with cytosines to amplify methylated DNA, and one set recognizes DNA with uracil or thymine to amplify unmethylated DNA. MSP using qPCR can also be performed to obtain quantitative rather than qualitative information about methylation.
- Miniprimer PCR: uses a thermostable polymerase (S-Tbr) that can extend from short primers ("smalligos") as short as 9 or 10 nucleotides. This method permits PCR targeting to smaller primer binding regions, and is used to amplify conserved DNA sequences, such as the 16S (or eukaryotic 18S) rRNA gene.[57]
- Multipleks ligatsiyaga bog'liq probni kuchaytirish (MLPA): permits amplifying multiple targets with a single primer pair, thus avoiding the resolution limitations of multiplex PCR (see below).
- Multiplex-PCR: consists of multiple primer sets within a single PCR mixture to produce amplicons of varying sizes that are specific to different DNA sequences. By targeting multiple genes at once, additional information may be gained from a single test-run that otherwise would require several times the reagents and more time to perform. Annealing temperatures for each of the primer sets must be optimized to work correctly within a single reaction, and amplicon sizes. That is, their base pair length should be different enough to form distinct bands when visualized by gel elektroforezi.
- Nanoparticle-Assisted PCR (nanoPCR): some nanoparticles (NPs) can enhance the efficiency of PCR (thus being called nanoPCR), and some can even outperform the original PCR enhancers. It was reported that quantum dots (QDs) can improve PCR specificity and efficiency. Single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) are efficient in enhancing the amplification of long PCR. Carbon nanopowder (CNP) can improve the efficiency of repeated PCR and long PCR, while rux oksidi, titanium dioksid and Ag NPs were found to increase the PCR yield. Previous data indicated that non-metallic NPs retained acceptable amplification fidelity. Given that many NPs are capable of enhancing PCR efficiency, it is clear that there is likely to be great potential for nanoPCR technology improvements and product development.[58][59]
- Nested PCR: increases the specificity of DNA amplification, by reducing background due to non-specific amplification of DNA. Two sets of primers are used in two successive PCRs. In the first reaction, one pair of primers is used to generate DNA products, which besides the intended target, may still consist of non-specifically amplified DNA fragments. The product(s) are then used in a second PCR with a set of primers whose binding sites are completely or partially different from and located 3' of each of the primers used in the first reaction. Nested PCR is often more successful in specifically amplifying long DNA fragments than conventional PCR, but it requires more detailed knowledge of the target sequences.
- Overlap-extension PCR yoki Splicing by overlap extension (SOEing) : a gen muhandisligi technique that is used to splice together two or more DNA fragments that contain complementary sequences. It is used to join DNA pieces containing genes, regulatory sequences, or mutations; the technique enables creation of specific and long DNA constructs. It can also introduce deletions, insertions or point mutations into a DNA sequence.[60][61]
- PAN-AC: uses isothermal conditions for amplification, and may be used in living cells.[62][63]
- miqdoriy PCR (qPCR): used to measure the quantity of a target sequence (commonly in real-time). It quantitatively measures starting amounts of DNA, cDNA, or RNA. miqdoriy PCR is commonly used to determine whether a DNA sequence is present in a sample and the number of its copies in the sample. Miqdor PCR has a very high degree of precision. Quantitative PCR methods use fluorescent dyes, such as Sybr Green, EvaGreen or florofor -containing DNA probes, such as TaqMan, to measure the amount of amplified product in real time. It is also sometimes abbreviated to RT-PCR (haqiqiy vaqt PCR) but this abbreviation should be used only for reverse transcription PCR. qPCR is the appropriate contractions for miqdoriy PCR (real-time PCR).
- Reverse Transcription PCR (RT-PCR ): for amplifying DNA from RNA. Teskari transkriptaz reverse transcribes RNK ichiga cDNA, which is then amplified by PCR. RT-PCR is widely used in ifodani profillashtirish, to determine the expression of a gene or to identify the sequence of an RNA transcript, including transcription start and termination sites. If the genomic DNA sequence of a gene is known, RT-PCR can be used to map the location of exons va intronlar in the gene. The 5' end of a gene (corresponding to the transcription start site) is typically identified by RACE-PCR (CDNA DNKlarining tez kuchayishi).
- RNase H ga bog'liq PCR (rhPCR): a modification of PCR that utilizes primers with a 3’ extension block that can be removed by a thermostable RNase HII enzyme. This system reduces primer-dimers and allows for multiplexed reactions to be performed with higher numbers of primers.[64]
- Single Specific Primer-PCR (SSP-PCR): allows the amplification of double-stranded DNA even when the sequence information is available at one end only. This method permits amplification of genes for which only a partial sequence information is available, and allows unidirectional genome walking from known into unknown regions of the chromosome.[65]
- Solid Phase PCR: encompasses multiple meanings, including Polony Amplification (where PCR colonies are derived in a gel matrix, for example), Bridge PCR[66] (primers are covalently linked to a solid-support surface), conventional Solid Phase PCR (where Asymmetric PCR is applied in the presence of solid support bearing primer with sequence matching one of the aqueous primers) and Enhanced Solid Phase PCR[67] (where conventional Solid Phase PCR can be improved by employing high Tm and nested solid support primer with optional application of a thermal 'step' to favour solid support priming).
- Suicide PCR: typically used in paleogenetika or other studies where avoiding false positives and ensuring the specificity of the amplified fragment is the highest priority. It was originally described in a study to verify the presence of the microbe Yersinia pestis in dental samples obtained from 14th Century graves of people supposedly killed by the plague during the medieval Qora o'lim epidemik.[68] The method prescribes the use of any primer combination only once in a PCR (hence the term "suicide"), which should never have been used in any positive control PCR reaction, and the primers should always target a genomic region never amplified before in the lab using this or any other set of primers. This ensures that no contaminating DNA from previous PCR reactions is present in the lab, which could otherwise generate false positives.
- Thermal asymmetric interlaced PCR (TAIL-PCR): for isolation of an unknown sequence flanking a known sequence. Within the known sequence, TAIL-PCR uses a nested pair of primers with differing annealing temperatures; a degenerate primer is used to amplify in the other direction from the unknown sequence.[69]
- Touchdown PCR (Step-down PCR): a variant of PCR that aims to reduce nonspecific background by gradually lowering the annealing temperature as PCR cycling progresses. The annealing temperature at the initial cycles is usually a few degrees (3–5 °C) above the Tm of the primers used, while at the later cycles, it is a few degrees (3–5 °C) below the primer Tm. The higher temperatures give greater specificity for primer binding, and the lower temperatures permit more efficient amplification from the specific products formed during the initial cycles.[70]
- Universal Fast Walking: for genome walking and genetic fingerprinting using a more specific 'two-sided' PCR than conventional 'one-sided' approaches (using only one gene-specific primer and one general primer—which can lead to artefactual 'noise')[71] by virtue of a mechanism involving lariat structure formation. Streamlined derivatives of UFW are LaNe RAGE (lariat-dependent nested PCR for rapid amplification of genomic DNA ends),[72] 5'RACE LaNe[73] and 3'RACE LaNe.[74]
Tarix

The heat-resistant enzymes that are a key component in polymerase chain reaction were discovered in the 1960s as a product of a microbial life form that lived in the superheated waters of Yellowstone ’s Mushroom Spring.[75]
A 1971 paper in the Molekulyar biologiya jurnali tomonidan Kjell Kleppe and co-workers in the laboratory of H. Gobind Xorana first described a method of using an enzymatic assay to replicate a short DNA template with primers in vitro.[76] However, this early manifestation of the basic PCR principle did not receive much attention at the time and the invention of the polymerase chain reaction in 1983 is generally credited to Kari Mullis.[77]

When Mullis developed the PCR in 1983, he was working in Emeryvil, Kaliforniya uchun Cetus Corporation, birinchilardan biri biotexnologiya companies, where he was responsible for synthesizing short chains of DNA. Mullis has written that he conceived the idea for PCR while cruising along the Tinch okean sohilidagi magistral one night in his car.[78] He was playing in his mind with a new way of analyzing changes (mutations) in DNA when he realized that he had instead invented a method of amplifying any DNA region through repeated cycles of duplication driven by DNA polymerase. Yilda Ilmiy Amerika, Mullis summarized the procedure: "Beginning with a single molecule of the genetic material DNA, the PCR can generate 100 billion similar molecules in an afternoon. The reaction is easy to execute. It requires no more than a test tube, a few simple reagents, and a source of heat."[79] DNA fingerprinting was first used for otalikni sinovdan o'tkazish 1988 yilda.[80]
Mullis was awarded the Kimyo bo'yicha Nobel mukofoti in 1993 for his invention, seven years after he and his colleagues at Cetus first put his proposal to practice.[81] Mullis's 1985 paper with R. K. Saiki and H. A. Erlich, “Enzymatic Amplification of β-globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia”—the polymerase chain reaction invention (PCR) – was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society in 2017.[82][1]
At the core of the PCR method is the use of a suitable DNK polimeraza able to withstand the high temperatures of >90 °C (194 °F) required for separation of the two DNA strands in the DNA double helix har biridan keyin takrorlash tsikl The DNA polymerases initially employed for in vitro experiments presaging PCR were unable to withstand these high temperatures.[1] So the early procedures for DNA replication were very inefficient and time-consuming, and required large amounts of DNA polymerase and continuous handling throughout the process.
The discovery in 1976 of Taq polimeraza —a DNA polymerase purified from the thermophilic bacterium, Thermus aquaticus, which naturally lives in hot (50 to 80 °C (122 to 176 °F)) environments[13] such as hot springs—paved the way for dramatic improvements of the PCR method. The DNA polymerase isolated from T. aquaticus is stable at high temperatures remaining active even after DNA denaturation,[14] thus obviating the need to add new DNA polymerase after each cycle.[2] This allowed an automated thermocycler-based process for DNA amplification.
Patent bo'yicha nizolar
The PCR technique was patented by Kari Mullis va tayinlangan Cetus Corporation, where Mullis worked when he invented the technique in 1983. The Taq polymerase enzyme was also covered by patents. There have been several high-profile lawsuits related to the technique, including an unsuccessful lawsuit brought by DuPont. The Swiss pharmaceutical company Hoffmann-La Roche purchased the rights to the patents in 1992 and currently[qachon? ] holds those that are still protected.
A related patent battle over the Taq polymerase enzyme is still ongoing in several jurisdictions around the world between Roche and Promega. The legal arguments have extended beyond the lives of the original PCR and Taq polymerase patents, which expired on March 28, 2005.[83]
Shuningdek qarang
Adabiyotlar
- ^ a b v d Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (December 1985). "Beta-globin genomik ketma-ketliklarini fermentativ ravishda kuchaytirish va o'roqsimon hujayrali anemiya diagnostikasi uchun cheklash joyini tahlil qilish". Ilm-fan. 230 (4732): 1350–4. Bibcode:1985Sci ... 230.1350S. doi:10.1126 / science.2999980. PMID 2999980.
- ^ a b Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. (1988 yil yanvar). "Termostabil DNK polimeraza bilan DNKning primer yo'naltirilgan fermentativ amplifikatsiyasi". Ilm-fan. 239 (4839): 487–91. Bibcode:1988Sci...239..487S. doi:10.1126/science.239.4839.487. PMID 2448875.
- ^ Enners, Edward; Porta, Angela R. (2012). "Determining Annealing Temperatures for Polymerase Chain Reaction". Amerika biologiya o'qituvchisi. 74 (4): 256–260. doi:10.1525/abt.2012.74.4.9. S2CID 86708426.
- ^ a b v d e f Ninfa, Alexander; Ballou, Devid; Benore, Marilee (2009). Biokimyo va biotexnologiya uchun asosiy laboratoriya yondashuvlari. United States: Wiley. 408-10 betlar. ISBN 978-0-47008766-4.
- ^ Cheng S, Fockler C, Barnes WM, Higuchi R (June 1994). "Effective amplification of long targets from cloned inserts and human genomic DNA". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 91 (12): 5695–9. Bibcode:1994PNAS...91.5695C. doi:10.1073/pnas.91.12.5695. PMC 44063. PMID 8202550.
- ^ Carr AC, Moore SD (2012). Lucia A (tahrir). "Robust quantification of polymerase chain reactions using global fitting". PLOS ONE. 7 (5): e37640. Bibcode:2012PLoSO ... 737640C. doi:10.1371 / journal.pone.0037640. PMC 3365123. PMID 22701526.
- ^ a b Jozef Sambruk va Devid V. Rassel (2001). Molecular Cloning: A Laboratory Manual (3-nashr). Cold Spring Harbor, NY: Cold Spring Harbor Laboratoriya matbuoti. ISBN 978-0-879-69576-7. Chapter 8: In vitro Amplification of DNA by the Polymerase Chain Reaction
- ^ "Polymerase Chain Reaction (PCR)". Milliy Biotexnologiya Axborot Markazi, AQSh Milliy Tibbiyot Kutubxonasi.
- ^ "PCR". Genetik fanlarni o'rganish markazi, Yuta universiteti.
- ^ Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI (May 2004). "Recent developments in the optimization of thermostable DNA polymerases for efficient applications". Biotexnologiyaning tendentsiyalari. 22 (5): 253–60. doi:10.1016/j.tibtech.2004.02.011. PMID 15109812.
- ^ Rychlik V, Spenser WJ, Rhoads RE (1990 yil noyabr). "Optimization of the annealing temperature for DNA amplification in vitro". Nuklein kislotalarni tadqiq qilish. 18 (21): 6409–12. doi:10.1093/nar/18.21.6409. PMC 332522. PMID 2243783.
- ^ a b Sharkey DJ, Scalice ER, Christy KG, Atwood SM, Daiss JL (May 1994). "Antibodies as thermolabile switches: high temperature triggering for the polymerase chain reaction". Bio / Technology. 12 (5): 506–9. doi:10.1038/nbt0594-506. PMID 7764710. S2CID 2885453.
- ^ a b Chien A, Edgar DB, Trela JM (September 1976). "Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus". Bakteriologiya jurnali. 127 (3): 1550–7. doi:10.1128/jb.127.3.1550-1557.1976. PMC 232952. PMID 8432.
- ^ a b Lawyer FC, Stoffel S, Saiki RK, Chang SY, Landre PA, Abramson RD, Gelfand DH (May 1993). "High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5' to 3' exonuclease activity". PCR Methods and Applications. 2 (4): 275–87. doi:10.1101/gr.2.4.275. PMID 8324500.
- ^ a b v d Schochetman G, Ou CY, Jones WK (December 1988). "Polymerase chain reaction". Yuqumli kasalliklar jurnali. 158 (6): 1154–7. doi:10.1093/infdis/158.6.1154. JSTOR 30137034. PMID 2461996.
- ^ Borman, Jon; Schuster, David; Li, Wu-bo; Jessee, Joel; Rashtchian, Ayoub (2000). "PCR from problematic templates" (PDF). Fokus. 22 (1): 10. Archived from asl nusxasi (PDF) 2017 yil 7 martda.
- ^ Bogetto, Prachi; Waidne, Lisa; Anderson, Holly (2000). "Helpful tips for PCR" (PDF). Fokus. 22 (1): 12. Archived from asl nusxasi (PDF) 2017 yil 7 martda.
- ^ Sarkar G, Kapelner S, Sommer SS (December 1990). "Formamide can dramatically improve the specificity of PCR". Nuklein kislotalarni tadqiq qilish. 18 (24): 7465. doi:10.1093/nar/18.24.7465. PMC 332902. PMID 2259646.
- ^ "Elektron PCR". NCBI – National Center for Biotechnology Information. Olingan 13 mart 2012.
- ^ Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI (2006). "Thermostable DNA Polymerases for a Wide Spectrum of Applications: Comparison of a Robust Hybrid TopoTaq to other enzymes". In Kieleczawa J (ed.). DNA Sequencing II: Optimizing Preparation and Cleanup. Jons va Bartlett. pp. 241–57. ISBN 978-0-7637-3383-4.
- ^ Pombert JF, Sistek V, Boissinot M, Frenette M (October 2009). "Evolutionary relationships among salivarius streptococci as inferred from multilocus phylogenies based on 16S rRNA-encoding, recA, secA, and secY gene sequences". BMC mikrobiologiyasi. 9: 232. doi:10.1186/1471-2180-9-232. PMC 2777182. PMID 19878555.
- ^ "Chemical Synthesis, Sequencing, and Amplification of DNA (class notes on MBB/BIO 343)". Arizona shtati universiteti. Arxivlandi asl nusxasi 1997 yil 9 oktyabrda. Olingan 29 oktyabr 2007.
- ^ Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (2009 yil aprel). "The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments" (PDF). Klinik kimyo. 55 (4): 611–22. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- ^ a b v d Garibyan L, Avashia N (March 2013). "Polymerase chain reaction". Tergov dermatologiyasi jurnali. 133 (3): 1–4. doi:10.1038 / jid.2013.1. PMC 4102308. PMID 23399825.
- ^ Shnell, S .; Mendoza, C. (October 1997). "Theoretical Description of the Polymerase Chain Reaction". Nazariy biologiya jurnali. 188 (3): 313–318. doi:10.1006/jtbi.1997.0473. PMID 9344735.
- ^ Shnell, S .; Mendoza, C. (21 February 1997). "Enzymological Considerations for the Theoretical Description of the Quantitative Competitive Polymerase Chain Reaction (QC-PCR)". Nazariy biologiya jurnali. 184 (4): 433–440. doi:10.1006/jtbi.1996.0283. ISSN 0022-5193. PMID 9082073.
- ^ Becker, Sven; Böger, Peter; Oehlmann, Ralfh; Ernst, Anneliese (1 November 2000). "PCR Bias in Ecological Analysis: a Case Study for Quantitative Taq Nuclease Assays in Analyses of Microbial Communities". Amaliy va atrof-muhit mikrobiologiyasi. 66 (11): 4945–4953. doi:10.1128/AEM.66.11.4945-4953.2000. ISSN 1098-5336. PMC 92404. PMID 11055948.
- ^ Solomon, Anthony W.; Peeling, Rosanna W.; Foster, Allen; Mabey, David C. W. (1 October 2004). "Diagnosis and Assessment of Trachoma". Klinik mikrobiologiya sharhlari. 17 (4): 982–1011. doi:10.1128/CMR.17.4.982-1011.2004. ISSN 0893-8512. PMC 523557. PMID 15489358.
- ^ Ramzy, Reda M.R. (April 2002). "Recent advances in molecular diagnostic techniques for human lymphatic filariasis and their use in epidemiological research". Tropik tibbiyot va gigiena qirollik jamiyatining operatsiyalari. 96: S225–S229. doi:10.1016/S0035-9203(02)90080-5. PMID 12055843.
- ^ Sachse, Konrad (2003). Saxse, Konrad; Frey, Joachim (eds.). "Specificity and Performance of Diagnostic PCR Assays". PCR Detection of Microbial Pathogens. Molekulyar biologiya usullari. Totova, Nyu-Jersi: Humana Press. 216: 3–29. doi:10.1385/1-59259-344-5:03. ISBN 978-1-59259-344-6. PMID 12512353.
- ^ Quill E (March 2008). "Medicine. Blood-matching goes genetic". Ilm-fan. 319 (5869): 1478–9. doi:10.1126/science.319.5869.1478. PMID 18339916. S2CID 36945291.
- ^ Tomar, Rukam (2010). Molecular Markers and Plant Biotechnology. Pitman Pura, New Delhi: New India Publishing Agency. p. 188. ISBN 978-93-80235-25-7.
- ^ a b v Cai HY, Caswell JL, Prescott JF (March 2014). "Nonculture molecular techniques for diagnosis of bacterial disease in animals: a diagnostic laboratory perspective". Veterinariya patologiyasi. 51 (2): 341–50. doi:10.1177/0300985813511132. PMID 24569613.
- ^ Salis AD (2009). "Applications in Clinical Microbiology". Real-Time PCR: Current Technology and Applications. Caister Academic Press. ISBN 978-1-904455-39-4.
- ^ Kwok S, Mack DH, Mullis KB, Poiesz B, Ehrlich G, Blair D, et al. (1987 yil may). "Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection". Virusologiya jurnali. 61 (5): 1690–4. doi:10.1128/jvi.61.5.1690-1694.1987. PMC 254157. PMID 2437321.
- ^ "Coronavirus: il viaggio dei test". Istituto Superiore di Sanità.
- ^ Barmoq, Xorst; von Koenig, Carl Heinz Wirsing (1996). Baron, Shomuil (tahr.) Tibbiy mikrobiologiya (4-nashr). Galveston, TX: Texas universiteti tibbiyot filiali Galveston. ISBN 978-0-96311721-2. PMID 21413270.
- ^ Yeh, Sylvia H.; Mink, ChrisAnna M. (2012). "Bordetella pertussis and Pertussis (Whooping Cough)". Netter's Infectious Diseases. Netter's Infectious Diseases. 11-14 betlar. doi:10.1016/B978-1-4377-0126-5.00003-3. ISBN 978-1-43770126-5.
- ^ Alonso A, Martín P, Albarrán C, García P, García O, de Simón LF, et al. (2004 yil yanvar). "Real-Time PCR Designs to Estimate Nuclear and Mitochondrial DNA Copy Number in Forensic and Ancient DNA Studies". Xalqaro sud ekspertizasi. 139 (2–3): 141–9. doi:10.1016/j.forsciint.2003.10.008. PMID 15040907.
- ^ Boehnke M, Arnheim N, Li H, Collins FS (July 1989). "Fine-structure genetic mapping of human chromosomes using the polymerase chain reaction on single sperm: experimental design considerations". Amerika inson genetikasi jurnali. 45 (1): 21–32. PMC 1683385. PMID 2568090.
- ^ Zhou YH, Zhang XP, Ebright RH (November 1991). "Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase". Nuklein kislotalarni tadqiq qilish. 19 (21): 6052. doi:10.1093/nar/19.21.6052. PMC 329070. PMID 1658751.
- ^ Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, et al. (1989 yil aprel). "DNKdagi har qanday nuqta mutatsiyasini tahlil qilish. Amplifikatsiya refrakter mutatsion tizimi (ARMS)". Nuklein kislotalarni tadqiq qilish. 17 (7): 2503–16. doi:10.1093 / nar / 17.7.2503. PMC 317639. PMID 2785681.
- ^ Stemmer WP, Crameri A, Ha KD, Brennan TM, Heyneker HL (oktyabr 1995). "Ko'p sonli oligodeoksiribonukleotidlardan gen va butun plazmidni bir bosqichli yig'ish". Gen. 164 (1): 49–53. doi:10.1016/0378-1119(95)00511-4. PMID 7590320.
- ^ Innis MA, Myambo KB, Gelfand DH, Brow MA (December 1988). "DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 85 (24): 9436–40. Bibcode:1988PNAS...85.9436I. doi:10.1073/pnas.85.24.9436. PMC 282767. PMID 3200828.
- ^ Pierce KE, Wangh LJ (2007). Linear-after-the-exponential polymerase chain reaction and allied technologies Real-time detection strategies for rapid, reliable diagnosis from single cells. Methods in Molecular Medicine. 132. 65-85 betlar. doi:10.1007/978-1-59745-298-4_7. ISBN 978-1-58829-578-1. PMID 17876077.
- ^ Krishnan M, Ugaz VM, Burns MA (October 2002). "PCR in a Rayleigh-Bénard convection cell". Ilm-fan. 298 (5594): 793. doi:10.1126/science.298.5594.793. PMID 12399582.
- ^ Priye A, Hassan YA, Ugaz VM (November 2013). "Microscale chaotic advection enables robust convective DNA replication". Analitik kimyo. 85 (21): 10536–41. doi:10.1021/ac402611s. PMID 24083802.
- ^ Schwartz JJ, Lee C, Shendure J (September 2012). "Accurate gene synthesis with tag-directed retrieval of sequence-verified DNA molecules". Tabiat usullari. 9 (9): 913–5. doi:10.1038/nmeth.2137. PMC 3433648. PMID 22886093.
- ^ Vincent M, Xu Y, Kong H (August 2004). "Helicase-ga bog'liq izotermik DNKni kuchaytirish". EMBO hisobotlari. 5 (8): 795–800. doi:10.1038 / sj.embor.7400200. PMC 1249482. PMID 15247927.
- ^ Chou Q, Russell M, Birch DE, Raymond J, Bloch W (April 1992). "Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications". Nuklein kislotalarni tadqiq qilish. 20 (7): 1717–23. doi:10.1093/nar/20.7.1717. PMC 312262. PMID 1579465.
- ^ Kellogg DE, Rybalkin I, Chen S, Mukhamedova N, Vlasik T, Siebert PD, Chenchik A (June 1994). "TaqStart Antibody: "hot start" PCR facilitated by a neutralizing monoclonal antibody directed against Taq DNA polymerase". Biotexnikalar. 16 (6): 1134–7. PMID 8074881.
- ^ San Millán RM, Martínez-Ballesteros I, Rementeria A, Garaizar J, Bikandi J (December 2013). "Online exercise for the design and simulation of PCR and PCR-RFLP experiments". BMC tadqiqotlari bo'yicha eslatmalar. 6: 513. doi:10.1186/1756-0500-6-513. PMC 4029544. PMID 24314313.
- ^ Zietkiewicz E, Rafalski A, Labuda D (March 1994). "Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification". Genomika. 20 (2): 176–83. doi:10.1006/geno.1994.1151. PMID 8020964.
- ^ Ochman H, Gerber AS, Hartl DL (November 1988). "Teskari polimeraza zanjiri reaktsiyasining genetik qo'llanilishi". Genetika. 120 (3): 621–3. PMC 1203539. PMID 2852134.
- ^ Mueller PR, Wold B (November 1989). "In vivo footprinting of a muscle specific enhancer by ligation mediated PCR". Ilm-fan. 246 (4931): 780–6. Bibcode:1989Sci...246..780M. doi:10.1126/science.2814500. PMID 2814500.
- ^ Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (September 1996). "Metilatsiyaga xos PCR: CpG orollari metilatsiyasining holati bo'yicha yangi PCR tahlillari". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 93 (18): 9821–6. Bibcode:1996 yil PNAS ... 93.9821H. doi:10.1073 / pnas.93.18.9821. PMC 38513. PMID 8790415.
- ^ Isenbarger TA, Finney M, Ríos-Velázquez C, Handelsman J, Ruvkun G (February 2008). "Miniprimer PCR, a new lens for viewing the microbial world". Amaliy va atrof-muhit mikrobiologiyasi. 74 (3): 840–9. doi:10.1128/AEM.01933-07. PMC 2227730. PMID 18083877.
- ^ Shen C, Yang W, Ji Q, Maki H, Dong A, Zhang Z (November 2009). "NanoPCR observation: different levels of DNA replication fidelity in nanoparticle-enhanced polymerase chain reactions". Nanotexnologiya. 20 (45): 455103. Bibcode:2009Nanot..20S5103S. doi:10.1088/0957-4484/20/45/455103. PMID 19822925. S2CID 3393115.
- ^ Shen, Cenchao (2013). "An Overview of Nanoparticle-Assisted Polymerase Chain Reaction Technology". An Overview of Nanoparticle‐Assisted Polymerase Chain Reaction Technology. US: Wiley-Blackwell Publishing Ltd. pp. 97–106. doi:10.1002/9781118451915.ch5. ISBN 9781118451915.
- ^ Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR (April 1989). "Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension". Gen. 77 (1): 61–8. doi:10.1016/0378-1119(89)90359-4. PMID 2744488.
- ^ Moller, Simon (2006). PCR: The Basics. US: Taylor & Francis Group. p. 144. ISBN 9780415355476.
- ^ David F, Turlotte E (November 1998). "[A method of isothermal gene amplification]" [An Isothermal Amplification Method]. Comptes Rendus de l'Académie des Sciences. Série III, Sciences de la Vie. 321 (11): 909–14. Bibcode:1998CRASG.321..909D. doi:10.1016/S0764-4469(99)80005-5. PMID 9879470.
- ^ Fabrice David (September–October 2002). "Utiliser les propriétés topologiques de l'ADN: une nouvelle arme contre les agents pathogènes" (PDF). Birlashma. Arxivlandi asl nusxasi (PDF) on 28 November 2007.(frantsuz tilida)
- ^ Dobosy JR, Rose SD, Beltz KR, Rupp SM, Powers KM, Behlke MA, Walder JA (avgust 2011). "RNase H-ga bog'liq PCR (rhPCR): takomillashtirilgan o'ziga xoslik va blokirovka qilingan ajratiladigan primerlardan foydalangan holda bitta nukleotid polimorfizmni aniqlash". BMC biotexnologiyasi. 11: 80. doi:10.1186/1472-6750-11-80. PMC 3224242. PMID 21831278.
- ^ Shyamala, V.; Ferro-Luzzi, Ames G. (1993). Single Specific Primer-Polymerase Chain Reaction (SSP-PCR) and Genome Walking. Molekulyar biologiya usullari. 15. 339-48 betlar. doi:10.1385/0-89603-244-2:339. ISBN 978-0-89603-244-6. PMID 21400290.
- ^ Bing DH, Boles C, Rehman FN, Audeh M, Belmarsh M, Kelley B, Adams CP (1996). "Bridge amplification: a solid phase PCR system for the amplification and detection of allelic differences in single copy genes". Genetic Identity Conference Proceedings, Seventh International Symposium on Human Identification. Arxivlandi asl nusxasi on 7 May 2001.
- ^ Khan Z, Poetter K, Park DJ (April 2008). "Enhanced solid phase PCR: mechanisms to increase priming by solid support primers". Analitik biokimyo. 375 (2): 391–3. doi:10.1016/j.ab.2008.01.021. PMID 18267099.
- ^ Raoult D, Aboudharam G, Crubézy E, Larrouy G, Ludes B, Drancourt M (November 2000). "Molecular identification by "suicide PCR" of Yersinia pestis as the agent of medieval black death". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 97 (23): 12800–3. Bibcode:2000PNAS...9712800R. doi:10.1073/pnas.220225197. PMC 18844. PMID 11058154.
- ^ Liu YG, Whittier RF (February 1995). "Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking". Genomika. 25 (3): 674–81. doi:10.1016/0888-7543(95)80010-J. PMID 7759102.
- ^ Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (July 1991). "'Touchdown' PCR to circumvent spurious priming during gene amplification". Nuklein kislotalarni tadqiq qilish. 19 (14): 4008. doi:10.1093/nar/19.14.4008. PMC 328507. PMID 1861999.
- ^ Myrick KV, Gelbart WM (February 2002). "Universal Fast Walking for direct and versatile determination of flanking sequence". Gen. 284 (1–2): 125–31. doi:10.1016/S0378-1119(02)00384-0. PMID 11891053.
- ^ "Full Text – LaNe RAGE: a new tool for genomic DNA flanking sequence determination".
- ^ Park DJ (January 2005). "A new 5' terminal murine GAPDH exon identified using 5'RACE LaNe". Molecular Biotechnology. 29 (1): 39–46. doi:10.1385/MB:29:1:39. PMID 15668518. S2CID 45702164.
- ^ Park DJ (April 2004). "3' RACE LaNe: a simple and rapid fully nested PCR method to determine 3'-terminal cDNA sequence". Biotexnikalar. 36 (4): 586–8, 590. doi:10.2144/04364BM04. PMID 15088375.
- ^ "Key ingredient in coronavirus tests comes from Yellowstone's lakes". Ilm-fan. 31 mart 2020 yil. Olingan 13 may 2020.
- ^ Kleppe K, Ohtsuka E, Kleppe R, Molineux I, Khorana HG (March 1971). "Studies on polynucleotides. XCVI. Repair replications of short synthetic DNA's as catalyzed by DNA polymerases". Molekulyar biologiya jurnali. 56 (2): 341–61. doi:10.1016/0022-2836(71)90469-4. PMID 4927950.
- ^ Rabinov, Pol (1996). Making PCR: A Story of Biotechnology. Chikago: Chikago universiteti matbuoti. ISBN 978-0-226-70146-2.
- ^ Mullis, Kary (1998). Dancing Naked in the Mind Field. Nyu-York: Pantheon kitoblari. ISBN 978-0-679-44255-4.
- ^ Mullis KB (April 1990). "The unusual origin of the polymerase chain reaction". Ilmiy Amerika. 262 (4): 56–61, 64–5. Bibcode:1990SciAm.262d..56M. doi:10.1038/scientificamerican0490-56. PMID 2315679.
- ^ Patidar M, Agrawal S, Parveen F, Khare P (2015). "Molecular insights of saliva in solving paternity dispute". Journal of Forensic Dental Sciences. 7 (1): 76–9. doi:10.4103/0975-1475.150325. PMC 4330625. PMID 25709326.
- ^ "Kary B. Mullis – Nobel Lecture: The Polymerase Chain Reaction".
- ^ "Chemical Breakthrough Awards 2017 mukofotlari uchun iqtiboslar". Kimyo tarixi bo'limi. Olingan 12 mart 2018.
- ^ "Advice on How to Survive the Taq Wars". GEN Genetic Engineering News – Biobusiness Channel. 26 (9). 2006 yil 1-may.
Tashqi havolalar
| Kutubxona resurslari haqida Polimeraza zanjiri reaktsiyasi |
- PCR Animation maxanim.com
- Full Form of PCR
- OpenPCR Open-source PCR thermalcycler project
- US Patent for PCR
- OpenWetWare
- What is PCR plateau effect? YouTube o'quv videosi
- GeneWarrior Online PCR Primer design tool
- History of the Polymerase Chain Reaction dan Smitson instituti arxivi
- Computer exercise. Design of PCR and PCR-RFLP experiments
