Olmos - Diamond - Wikipedia
| Olmos | |
|---|---|
 Ushbu qo'pol olmos kristalining matritsadagi biroz noto'g'ri shakllangan oktahedral shakli mineralga xosdir. Uning porloq yuzlari ham bu kristalning asosiy qatlamdan ekanligini ko'rsatadi. | |
| Umumiy | |
| Turkum | Mahalliy minerallar |
| Formula (takroriy birlik) | C |
| Strunz tasnifi | 1. MB 10a |
| Dana tasnifi | 1.3.6.1 |
| Kristalli tizim | Kubik |
| Kristal sinf | Geksoktaedral (m3m) H-M belgisi: (4 / m 3 2 / m) |
| Kosmik guruh | Fd3m (№ 227) |
| Tuzilishi | |
| Jmol (3D) | Interaktiv rasm |
| Identifikatsiya | |
| Formula massasi | 12.01 g / mol |
| Rang | Odatda sariq, jigarrang yoki kul rangdan rangsizgacha. Ko'pincha ko'k, yashil, qora, shaffof oq, pushti, binafsha, to'q sariq, binafsha va qizil. |
| Kristall odat | Oktahedral |
| Tvinnizatsiya | Shpinel qonuni keng tarqalgan ("makle" hosil qiladi) |
| Ajratish | 111 (to'rt yo'nalishda mukammal) |
| Singan | Noqonuniy / notekis |
| Mohs o'lchovi qattiqlik | 10 (aniqlovchi mineral) |
| Yorqinlik | Adamantin |
| Yo'l | Rangsiz |
| Diafanlik | Shaffof shaffofdan shaffofgacha |
| O'ziga xos tortishish kuchi | 3.52±0.01 |
| Zichlik | 3.5–3.53 g / sm3 |
| Polsha porlashi | Adamantin |
| Optik xususiyatlari | Izotropik |
| Sinishi ko'rsatkichi | 2.418 (500 nm da) |
| Birjalikni buzish | Yo'q |
| Pleoxroizm | Yo'q |
| Tarqoqlik | 0.044 |
| Erish nuqtasi | Bosimga bog'liq |
| Adabiyotlar | [1][2] |
Olmos a uglerod elementining qattiq shakli uning atomlari a kristall tuzilishi deb nomlangan olmos kubik. Da xona harorati va bosimi, deb nomlanuvchi uglerodning yana bir qattiq shakli grafit bo'ladi kimyoviy jihatdan barqaror uglerod shakli, ammo olmos deyarli hech qachon unga aylanmaydi. Olmos eng yuqori darajaga ega qattiqlik va issiqlik o'tkazuvchanligi har qanday tabiiy materialning xususiyatlari, kesish va polishing asboblari kabi yirik sanoat dasturlarida qo'llaniladi. Buning sababi ham ular olmos anvil hujayralari materiallarni Yerning tubida joylashgan bosimlarga duchor qilishi mumkin.
Olmosdagi atomlarning joylashishi o'ta qattiq bo'lganligi sababli, nopoklikning bir nechta turlari uni ifloslantirishi mumkin (ikkita istisno bor va azot ). Kichik raqamlar nuqsonlar yoki aralashmalar (panjara atomlarining har millioniga taxminan bir) olmos ko'k (bor), sariq (azot), jigarrang (nuqsonlar), yashil (nurlanish ta'sirida), binafsha, pushti, to'q sariq yoki qizil ranglarda. Olmos ham nisbatan yuqori optik dispersiya (turli rangdagi yorug'likni tarqatish qobiliyati).
Ko'pgina tabiiy olmoslarning yoshi 1 milliarddan 3,5 milliard yilgacha. Ularning aksariyati Yerdagi 150 dan 250 kilometrgacha (93 va 155 milya) chuqurlikda hosil bo'lgan mantiya Bir necha kishi 800 kilometr (500 milya) chuqurlikdan kelgan bo'lsa-da. Yuqori bosim va harorat ostida uglerod o'z ichiga olgan suyuqliklar turli minerallarni eritib, ularni olmos bilan almashtirdi. Yaqinda (o'n-yuzlab million yil oldin) ular sirtga ko'tarildi vulqon otilishi va saqlangan magmatik jinslar sifatida tanilgan kimberlitlar va lamproitlar.
Sintetik olmoslar yuqori bosim va harorat ostida yuqori toza ugleroddan yoki dan etishtirish mumkin uglevodorod gaz bilan kimyoviy bug 'cho'kmasi (KVH). Olmoslarga taqlid qilish kabi materiallardan ham tayyorlanishi mumkin kubik zirkoniya va kremniy karbid. Tabiiy, sintetik va taqlid olmoslari odatda optik texnika yoki issiqlik o'tkazuvchanlik o'lchovlari yordamida ajralib turadi.
Moddiy xususiyatlar
Olmos - sof uglerodning qattiq shakli, uning atomlari kristallga joylashtirilgan. Qattiq uglerod turli shakllarda keladi allotroplar kimyoviy bog'lanish turiga qarab. Ikki eng keng tarqalgan sof uglerodning allotroplari olmos va grafit. Grafitda bog'lanishlar sp2 orbital duragaylar va atomlar tekislikda hosil bo'lib, ularning har biri 120 graduslik masofada uchta eng yaqin qo'shnilar bilan bog'langan. Olmosda ular sp3 va atomlar tetraedra hosil qiladi, ularning har biri to'rtta eng yaqin qo'shnilariga bog'langan.[3][4] Tetraedralar qattiq, bog'lanishlar mustahkam va ma'lum bo'lgan barcha moddalardan olmos birligi uchun eng katta miqdordagi atomlarga ega, shuning uchun ham eng qiyin, ham eng kichik siqiladigan.[5][6] Shuningdek, u tabiiy olmos tarkibidagi kubometr uchun 3150 dan 3530 kilogrammgacha (suv zichligidan uch baravar ko'p) va 3520 kg / m gacha bo'lgan zichlikka ega.3 toza olmosda.[1] Grafitda eng yaqin qo'shnilar o'rtasidagi aloqalar yanada kuchliroq, ammo samolyotlar orasidagi bog'lanish zaif, shuning uchun samolyotlar osongina bir-biridan o'tib ketishi mumkin. Shunday qilib, grafit olmosdan ancha yumshoqroq. Biroq, kuchli bog'lanishlar grafitni kamroq yonuvchan qiladi.[7]
Olmos materialning ajoyib jismoniy xususiyatlari tufayli ko'plab maqsadlarda ishlatilgan. Ma'lum bo'lgan barcha moddalar orasida bu eng qiyin va eng kam siqiladigan hisoblanadi. U eng yuqori darajaga ega issiqlik o'tkazuvchanligi va eng yuqori ovoz tezligi. Uning yopishqoqligi va ishqalanishi past, va uning koeffitsienti issiqlik kengayishi juda past. Uning optik shaffofligi uzoq infraqizil chuqurgacha ultrabinafsha va u yuqori optik dispersiya. Bundan tashqari, u yuqori elektr qarshiligiga ega. U kimyoviy jihatdan inert, aksariyat korroziv moddalar bilan reaksiyaga kirishmaydi va mukammal biologik muvofiqlikka ega.[8]
Termodinamika

Grafit va olmos o'rtasida o'tish uchun muvozanat bosimi va harorat sharoitlari nazariy va eksperimental ravishda yaxshi tasdiqlangan. Bosim o'rtasida chiziqli ravishda o'zgaradi 1.7 GPa da 0 K va 12 GPa da 5000 K (olmos / grafit / suyuqlik) uch ochko ).[9][10]Biroq, fazalar ushbu yo'nalish bo'yicha birgalikda yashashlari mumkin bo'lgan keng mintaqaga ega. Da normal harorat va bosim, 20 ° C (293 K) va 1 ta standart atmosfera (0,10 MPa), uglerodning barqaror fazasi grafit, ammo olmos metastable va uning grafitga aylanish darajasi juda kam.[6] Biroq, taxminan yuqori haroratlarda 4500 K, olmos tezda grafitga aylanadi. Grafitni olmosga tez aylantirish muvozanat chizig'idan ancha yuqori bosimlarni talab qiladi: at 2000 K, bosim 35 GPa kerak.[9]
Uch nuqta ustida olmosning erish nuqtasi bosim ortishi bilan asta sekin o'sib boradi; ammo yuzlab GPa bosimida u kamayadi.[11] Yuqori bosimlarda, kremniy va germaniy BC8ga ega tanaga yo'naltirilgan kub kristalli struktura va shunga o'xshash struktura yuqori bosim ostida uglerod uchun bashorat qilinadi. Da 0 K, o'tish sodir bo'lishi taxmin qilinmoqda 1100 GPa.[12]
Ilmiy jurnaldagi maqolada chop etilgan tadqiqot natijalari Tabiat 2010 yilda yuqori bosim va haroratda (taxminan 10 million atmosfera yoki 1 TPa va 50 000 ° S) olmos metall suyuqlik sifatida o'zini tutishini taxmin qilmoqda. Buning uchun zarur bo'lgan o'ta og'ir sharoitlar mavjud gaz gigantlari ning Neptun va Uran. Ikkala sayyora taxminan 10 foiz ugleroddan tashkil topgan va ular tarkibida suyuq uglerodli okean mavjud bo'lishi mumkin. Ko'p miqdordagi metall suyuqlik magnit maydonga ta'sir qilishi mumkinligi sababli, bu nima uchun ikki sayyoraning geografik va magnit qutblari bir-biriga mos kelmasligi haqida tushuntirish bo'lib xizmat qilishi mumkin.[13][14]
Kristal tuzilishi

Olmosning eng keng tarqalgan kristalli tuzilishi deyiladi olmos kubik. U shakllangan birlik hujayralari (rasmga qarang) bir-biriga yig'ilib. Rasmda 18 ta atom bo'lsa-da, har bir burchak atomini sakkiz birlik hujayra va yuzning markazidagi har bir atomni ikkitasi bo'lishadi, shuning uchun bir birlik hujayrada jami sakkiz atom mavjud.[15] Birlik katakchasining har bir tomoni 3,57 ga tengangstromlar uzunligi bo'yicha.[16]
Olmos kubikli panjarani ikkita o'zaro ta'sirlashuvchi deb hisoblash mumkin yuzga yo'naltirilgan kub kubik hujayra bo'ylab diagonali 1/4 ga siljigan panjaralar yoki har bir panjara nuqtasi bilan bog'langan ikkita atomli bitta panjara kabi.[16] A dan ko'rilgan <1 1 1> kristalografik yo'nalish, u takrorlanadigan ABCABC ... naqshiga joylashtirilgan qatlamlardan hosil bo'ladi. Olmos ABAB ... tuzilishini ham hosil qilishi mumkin, bu olti burchakli olmos yoki lonsdaleite, ammo bu juda kam tarqalgan va har xil sharoitlarda kubik ugleroddan hosil bo'ladi.[17]
Kristall odat

Olmos ko'pincha sodir bo'ladi euhedral yoki yumaloq oktaedra va egizak sifatida tanilgan oktaedra maklar. Olmosning kristalli tuzilishi atomlarning kubik tartibiga ega bo'lgani uchun ular ko'p qirralar a ga tegishli kub, oktaedr, rombikosidodekaedr, tetrakis olti qirrasi yoki disdyakis dodecahedron. Kristallar yumaloq va ifodasiz qirralarga ega bo'lishi mumkin va cho'zilishi mumkin. Olmos (ayniqsa, yuzlari yumaloq, dumaloq), odatda qoplamali holda topiladi nyf, saqichga o'xshash shaffof bo'lmagan teri.[18]
Ba'zi olmoslarda shaffof bo'lmagan tolalar mavjud. Ular deb nomlanadi shaffof emas agar tolalar aniq substratdan o'ssa yoki tolali agar ular butun kristalni egallasa. Ularning ranglari sariqdan yashil yoki kul ranggacha, ba'zida bulutga o'xshash oqdan kul ranggacha aralashmalarga ega. Ularning eng keng tarqalgan shakli kuboidaldir, lekin ular oktaedra, dodekaedra, makl yoki birlashgan shakllarni ham hosil qilishi mumkin. Tuzilishi 1 dan 5 mikrongacha bo'lgan ko'plab aralashmalarning natijasidir. Ushbu olmoslar, ehtimol, kimberlit magmasida hosil bo'lgan va uchuvchi moddalardan namuna olgan.[19]
Olmos shuningdek, polikristalli agregatlar hosil qilishi mumkin. Kabi nomlar bilan ularni guruhlarga ajratishga urinishlar bo'lgan boart, balalar, styuartit va ramzit, ammo keng miqyosda qabul qilingan mezonlar to'plami mavjud emas.[19] Karbonado, olmos donalari bo'lgan tur sinterlangan (issiqlik va bosim ta'sirida erimay eritilgan), qora rangga ega va bitta kristalli olmosdan qattiqroq.[20] Hech qachon vulqon toshida kuzatilmagan. Uning kelib chiqishi uchun ko'plab nazariyalar mavjud, shu jumladan yulduz shakllanishi, ammo yakdillik yo'q.[19][21][22]
Mexanik xususiyatlari
Qattiqlik

Olmos har ikkalasida ham ma'lum bo'lgan eng qiyin tabiiy materialdir Vikers miqyosi va Mohs o'lchovi. Olmosning boshqa materiallarga nisbatan katta qattiqligi qadim zamonlardan beri ma'lum bo'lgan va uning nomi manbai hisoblanadi. Bu uning cheksiz qattiq, buzilmas yoki chizilmasligini anglatmaydi.[23] Darhaqiqat, olmosni boshqa olmoslar tirnashi mumkin[24] kabi yumshoq materiallar bilan vaqt o'tishi bilan eskirgan vinil yozuvlar.[25]
Olmosning qattiqligi uning tozaligiga, kristalli mukammalligi va yo'nalishiga bog'liq: qusursiz, sof kristallar uchun qattiqlik yuqori <111> yo'nalish (kubik olmos panjarasining eng uzun diagonali bo'ylab).[26] Shu sababli, ba'zi bir olmoslarni boshqa materiallar bilan chizish mumkin bo'lishi mumkin, masalan bor nitridi, eng qiyin olmoslarni faqat boshqa olmoslar tirnalishi mumkin va nanokristalli olmos agregatlari.
Olmosning qattiqligi uning qimmatbaho toshga mos kelishiga yordam beradi. Uni faqat boshqa olmoslar tirnashi mumkinligi sababli, u o'zining jilosini juda yaxshi saqlaydi. Boshqa qimmatbaho toshlardan farqli o'laroq, u chizishga chidamliligi tufayli kundalik kiyinishga juda mos keladi, ehtimol bu uning taniqli marvarid sifatida mashhur bo'lishiga yordam beradi. nishon yoki nikoh uzuklari, ko'pincha har kuni kiyiladi.
Eng qiyin tabiiy olmoslar asosan kelib chiqadi Kopeton va Bingara da joylashgan maydonlar Yangi Angliya maydon Yangi Janubiy Uels, Avstraliya. Ushbu olmoslar odatda kichikdir, oktaedrani yarimo'tkazish uchun mukammal va boshqa olmoslarni silliqlash uchun ishlatiladi. Ularning qattiqligi kristall o'sishi bir bosqichli kristall o'sishi bo'lgan shakl. Aksariyat olmoslarning ko'pi o'sish bosqichlarining ko'proq dalillarini namoyish etadi, ular kristallik panjarasida inklüzyonlar, nuqsonlar va nuqsonli samolyotlar ishlab chiqaradi, bularning hammasi ularning qattiqligiga ta'sir qiladi. Muntazam olmoslarni yuqori bosim va yuqori harorat kombinatsiyasi ostida ishlov berish orqali qattiqlik o'lchagichlarida ishlatiladigan olmoslardan qiyinroq olmoslar ishlab chiqarish mumkin.[27]
Qattiqlik
Qattiqligicha bir oz bog'liq yana bir mexanik xususiyatdir qattiqlik, bu materialning kuchli ta'sirdan xalos bo'lishiga qarshi turish qobiliyati. The qattiqlik tabiiy olmos 7,5-10 ga teng bo'lganMPa · M1/2.[28][29] Ushbu qiymat boshqa keramika materiallari bilan taqqoslaganda yaxshi, ammo odatda 100 dan ortiq toklilikni namoyish etadigan muhandislik qotishmalari kabi ko'plab muhandislik materiallari bilan taqqoslaganda yomon MPa · m1/2. Har qanday materialda bo'lgani kabi, olmosning makroskopik geometriyasi ham uning sinishga chidamliligiga yordam beradi. Olmos a dekolte tekisligi va shuning uchun ba'zi yo'nalishlarda boshqalarga qaraganda mo'rtroq. Olmos to'sarlari yuzni burish oldidan ba'zi toshlarni yorish uchun ushbu xususiyatdan foydalaning.[30] "Ta'sirga chidamlilik" sintetik sanoat olmoslarining sifatini o'lchashning asosiy ko'rsatkichlaridan biridir.
Hosildorlik kuchi
Olmosning siqilish kuchi 130-140 gacha GPa.[31] Bu juda yuqori qiymat, olmosning qattiqligi va shaffofligi bilan bir qatorda, bunga sabab bo'ladi olmos anvil hujayralar yuqori bosimli tajribalar uchun asosiy vositadir.[32] Ushbu anvillar bosimga duch kelishdi 600 GPa.[33] Nanokristalli olmos bilan ancha yuqori bosimlar bo'lishi mumkin.[32][34]
Elastiklik va tortishish kuchi
Odatda, katta miqdordagi olmos kristalini kuchlanish yoki egilish bilan deformatsiyalashga urinish mo'rt sinishga olib keladi. Biroq, bitta kristalli olmos nanometrli simlar yoki ignalar shaklida bo'lganda (~ 100-300) diametri nanometr), ular elastik ravishda 9 foiz tortishish kuchi bilan uzilib qolishi mumkin,[35] ning maksimal mahalliy kuchlanish kuchlanishi bilan -99 dan 98 GPa gacha, ushbu material uchun nazariy chegaraga juda yaqin.[36]
Elektr o'tkazuvchanligi
Boshqa ixtisoslashtirilgan dasturlar ham mavjud yoki ishlab chiqilmoqda, jumladan foydalanish kabi yarim o'tkazgichlar: biroz ko'k olmos eng yaxshi olmoslardan farqli o'laroq tabiiy yarimo'tkazgichlardir elektr izolyatorlari. Supero'tkazuvchilar va ko'k rang bor aralashmasidan kelib chiqadi. Bor olmos panjarasidagi uglerod atomlarini o'rnini bosadi va teshik ochadi valentlik diapazoni.[37]
Jiddiy o'tkazuvchanlik odatda nominalda kuzatiladi bekor qilinmagan tomonidan yetishtirilgan olmos kimyoviy bug 'cho'kmasi. Ushbu o'tkazuvchanlik vodorod bilan bog'liq bo'lgan sirt ustida adsorbsiyalangan turlar bilan bog'liq bo'lib, uni yo'q qilish mumkin tavlash yoki boshqa sirt muolajalari.[38][39]
2020-yilgi bir nashrda ta'kidlanishicha, olmosning juda nozik ignalari elektr qarshiligini normal (5,6 eV tarmoqli oralig'i) dan nolga qadar selektiv valf deformatsiyasi bilan o'zgartirishi mumkin.[40]
Yuzaki xususiyat
Olmos tabiiy lipofil va hidrofob degan ma'noni anglatadi, bu olmos yuzasini suv bilan ho'llash mumkin emas, lekin osongina namlanib, yog'ga yopishib qolishi mumkin. Ushbu xususiyatdan sintetik olmoslarni ishlab chiqarishda yog 'yordamida olmoslarni olish uchun foydalanish mumkin. Biroq, olmosli yuzalar ma'lum ionlar bilan kimyoviy o'zgartirilganda, ular shunday bo'lishi kutilmoqda hidrofilik ular bir necha qatlamlarni barqarorlashtirishi mumkin suvli muz da inson tanasining harorati.[41]
Olmos yuzasi qisman oksidlangan. Oksidlangan sirtni vodorod oqimi ostida issiqlik bilan ishlov berish orqali kamaytirish mumkin. Ya'ni, bu issiqlik bilan ishlov berish kislorod o'z ichiga olgan funktsional guruhlarni qisman olib tashlaydi. Ammo olmos (sp3C) atmosfera bosimi ostida yuqori haroratga (taxminan 400 ° C (752 ° F) yuqori) nisbatan beqaror. Tuzilishi asta-sekin sp ga aylanadi2Ushbu haroratdan yuqori S. Shunday qilib, olmoslar ushbu harorat ostida kamaytirilishi kerak.[42]
Kimyoviy barqarorlik
Xona haroratida olmos kuchli kislotalar va asoslarni o'z ichiga olgan biron bir kimyoviy reagent bilan reaksiyaga kirishmaydi.
Sof kislorod atmosferasida olmos an bor ateşleme nuqtasi u 690 ° C (1,274 ° F) dan 840 ° C (1,540 ° F) gacha; kichikroq kristallar osonroq kuyishga moyil. U haroratni qizildan oq issiqgacha oshiradi va xira ko'k olov bilan yonadi va issiqlik manbai chiqarilgandan keyin ham yonishda davom etadi. Aksincha, havoda yonish issiqlik ketishi bilanoq to'xtaydi, chunki kislorod azot bilan suyultiriladi. Tiniq, beg'ubor, shaffof olmos to'liq karbonat angidridga aylanadi; har qanday aralashmalar kul bo'lib qoladi.[43] Olmosni kesishdan hosil bo'ladigan issiqlik olovni qo'zg'atmaydi,[44] va sigareta ham zajigalka bo'lmaydi,[45] ammo uy yong'inlari va portlovchi mash'alalar etarlicha issiq. Zargarlar metallni olmosli uzukka shakllantirishda ehtiyot bo'lishlari kerak.[46]
Tegishli don o'lchamidagi olmos kukuni (50 atrofida) mikron) olovdan otashdan keyin uchqunli dush bilan yonadi. Binobarin, pirotexnika kompozitsiyalari asoslangan sintetik olmos kukun tayyorlanishi mumkin. Natijada paydo bo'lgan uchqunlar odatdagi qizil-to'q sariq rangga ega bo'lib, ularni ko'mir bilan taqqoslash mumkin, ammo ularning yuqori zichligi bilan izohlanadigan juda chiziqli traektoriyani ko'rsatadi.[47] Olmos shuningdek, 700 ° C (1,292 ° F) dan yuqori bo'lgan ftorli gaz bilan reaksiyaga kirishadi.
Rang


Olmos keng bandgap ning 5.5 eV chuqurga mos keladi ultrabinafsha to'lqin uzunligi 225 nanometrlar. Bu shuni anglatadiki, sof olmos ko'zga ko'rinadigan yorug'likni o'tkazishi va tiniq rangsiz kristal bo'lib ko'rinishi kerak. Olmosdagi ranglar panjara qusurlari va aralashmalaridan kelib chiqadi. Olmos kristalli panjarasi juda kuchli va faqat atomlari azot, bor va vodorod o'sish paytida olmosga sezilarli kontsentratsiyalarda (atom foiziga qadar) kiritilishi mumkin. O'tish metallari nikel va kobalt sintetik olmosni yuqori bosimli yuqori haroratli usullar bilan ko'paytirish uchun odatda ishlatiladigan, olmosda alohida atomlar sifatida aniqlangan; maksimal kontsentratsiya nikel uchun 0,01% ni tashkil qiladi[48] kobalt uchun esa undan ham kamroq. Olmosga deyarli har qanday elementni ion implantatsiyasi bilan kiritish mumkin.[49]
Azot qimmatbaho olmos tarkibidagi eng keng tarqalgan nopoklikdir va olmos tarkibidagi sariq va jigarrang ranglar uchun javobgardir. Bor rang ko'k rang uchun javobgardir.[50] Olmosdagi rang ikkita qo'shimcha manbaga ega: nurlanish (odatda alfa zarralari orqali), bu yashil olmos rangini keltirib chiqaradi va plastik deformatsiya olmos kristalli panjarasining Plastmassa deformatsiyasi - ba'zi jigarrang ranglarning sababi[51] va ehtimol pushti va qizil olmoslar.[52] Noyobligini oshirish maqsadida sariq olmosdan keyin jigarrang, rangsiz, so'ngra ko'k, yashil, qora, pushti, to'q sariq, binafsha va qizil ranglar turadi.[30] "Qora", yoki Karbonado, olmoslar chindan ham qora emas, aksincha toshlarga qorong'i ko'rinishni beradigan ko'plab qorong'u qo'shimchalarni o'z ichiga oladi. Rangli olmos tarkibida iflosliklar yoki tarkibiy nuqsonlar rang berishga olib keladi, toza yoki deyarli toza olmoslar shaffof va rangsizdir. Aksariyat olmos aralashmalari tarkibidagi uglerod atomini almashtiradi kristall panjara deb nomlanuvchi uglerod etishmovchiligi. Eng keng tarqalgan najosat azot mavjud bo'lgan azotning turi va kontsentratsiyasiga qarab engil va kuchli sariq rangga olib keladi.[30] The Amerikaning gemologik instituti (GIA) quyi to'yinganlikdagi sariq va jigarrang olmoslarni olmos sifatida tasniflaydi normal rang oralig'i, va "D" (rangsiz) dan "Z" (och sariq) gacha bo'lgan baho o'lchovini qo'llaydi. Moviy kabi boshqa rangdagi olmoslar deyiladi chiroyli rangli olmos va boshqa baholash shkalasi ostiga tushadi.[30]
2008 yilda, Wittelsbach Diamond, 35,56 karat (7,122 g) ko'k olmos bir paytlar Ispaniya qiroliga tegishli bo'lib, Christie's kim oshdi savdosida 24 million dollardan oshdi.[53] 2009 yil may oyida 7,03 karat (1,406 g) ko'k olmos olmos kimoshdi savdosida 10,5 million shveytsariya franki (6,97 million evro yoki o'sha paytda 9,5 million AQSh dollari) ga sotilganda, karat uchun to'langan eng yuqori narxni oldi.[54] Shu bilan birga, o'sha yilgi rekord ham qayd etilgan: 2009 yil 1 dekabrda Gonkongda 5 karatli (1,0 g) jonli pushti olmos 10,8 million dollarga sotilgan.[55]
Identifikatsiya
Olmoslarni yuqori issiqlik o'tkazuvchanligi bilan aniqlash mumkin (900–2320 Vt · m−1· K−1).[56] Ularning balandligi sinish ko'rsatkichi shuningdek, indikativdir, ammo boshqa materiallar shunga o'xshash sinishi mumkin. Olmos shishani kesadi, ammo bu olmosni ijobiy aniqlamaydi, chunki kvarts singari boshqa materiallar ham shisha ustida joylashgan Mohs o'lchovi va uni kesishi ham mumkin. Olmos boshqa olmoslarni qirib tashlashi mumkin, ammo bu toshlardan biriga yoki ikkalasiga ham zarar etkazishi mumkin. Qattiqlik sinovlari potentsial halokatli xususiyatga ega bo'lganligi sababli amaliy gemologiyada kamdan kam qo'llaniladi.[57] Olmosning o'ta qattiqligi va yuqori qiymati shundan iboratki, qimmatbaho toshlar odatdagidek asta-sekin silliqlanadi, anchagina an'anaviy texnika va tafsilotlarga ko'proq e'tibor boshqa qimmatbaho toshlarga nisbatan qo'llaniladi;[58] natijada favqulodda qirralarning qirralari o'ta tekis, juda silliqlangan yuzlar paydo bo'ladi. Olmoslar shuningdek, juda yuqori sindirish ko'rsatkichiga va juda yuqori dispersiyaga ega. Birgalikda, bu omillar silliqlangan olmosning umumiy ko'rinishiga va aksariyatiga ta'sir qiladi diamantalar hali ham mahoratli foydalanishga tayanamiz lupa (lupa) olmoslarni "ko'z bilan" aniqlash uchun.[59]
Geologiya
Olmos juda kam uchraydi, ularning kontsentratsiyasi ko'p miqdordagi milliard toshga to'g'ri keladi.[19] 20-asrga qadar ko'pgina olmoslar topilgan allyuvial yotqiziqlar. Bo'sh olmoslar mavjud va qadimgi bo'ylab ham mavjud qirg'oq, bu erda ularning kattaligi va zichligi tufayli ular to'planishga moyil.[60]:149 Kamdan kam hollarda ular topilgan muzlikgacha (xususan. ichida Viskonsin va Indiana ), ammo bu konlar tijorat sifatiga ega emas.[60]:19 Ushbu depozit turlari mahalliy magmatiklardan olingan bosqinlar orqali ob-havo va transport tomonidan shamol yoki suv.[61]
Olmoslarning aksariyati Yer mantiyasi, va ushbu bo'limning ko'p qismida ushbu olmoslar muhokama qilinadi. Biroq, boshqa manbalar mavjud. Yer qobig'ining ba'zi bloklari yoki terranlar, etarlicha chuqur ko'milgan, chunki qobiq qalinlashgan va ular tajribaga ega bo'lgan ultra yuqori bosimli metamorfizm. Ular teng ravishda taqsimlangan mikro olmoslar magma orqali tashish belgisi yo'q. Bundan tashqari, meteoritlar erga tushganda, zarba to'lqini etarli darajada yuqori harorat va bosim hosil qilishi mumkin mikro olmoslar va nanodiamonds shakllantirmoq.[61] Qadimgi zarb kraterlari ko'rsatkichi sifatida zarbalar tipidagi mikrodiamondlardan foydalanish mumkin.[62] Popigai krateri Rossiyada dunyodagi eng katta olmos koni bo'lishi mumkin, u trillionlab karat bilan baholanadi va asteroid ta'sirida hosil bo'ladi.[63]
Oddiy noto'g'ri tushuncha shundaki, olmos juda siqilgan holda hosil bo'ladi ko'mir. Ko'mir ko'milgan tarixgacha bo'lgan o'simliklardan hosil bo'ladi va eskirgan olmoslarning aksariyati birinchisiga qaraganda ancha qadimiyroqdir quruqlikdagi o'simliklar. Olmos ko'mirdan hosil bo'lishi mumkin subduktsiya zonalari, ammo shu tarzda hosil bo'lgan olmoslar kamdan-kam uchraydi va uglerod manbai ehtimoli katta karbonat cho'kindilarda toshlar va organik uglerod[64][65]
Yuzaki taqsimlash
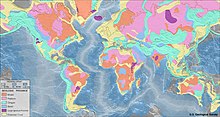
Olmoslar Yer yuzida bir tekis taqsimlanmagan. Klifford qoidasi deb nomlanuvchi bosh qoida, ular deyarli har doim kimberlitlarda eng qadimgi qismida joylashganligini aytadi. kratonlar, tipik yoshi 2,5 ga teng bo'lgan qit'alarning barqaror yadrolari milliard yil yoki undan ko'proq.[61][66]:314 Biroq, istisnolar mavjud. The Argil olmos koni yilda Avstraliya, og'irligi bo'yicha dunyodagi eng yirik olmos ishlab chiqaruvchisi a mobil kamar, shuningdek, an orogenik kamar,[67] siqilish tektonikasiga uchragan markaziy kraton atrofini zaifroq zonasi. Kimberlite o'rniga xost jinsi hisoblanadi lamproit. Iqtisodiy jihatdan foydasiz bo'lgan olmosli lamproitlar AQSh, Hindiston va Avstraliyada ham uchraydi.[61] Bundan tashqari, Wawa kamari yilda Superior viloyati Kanada va mikro olmoslar Yaponiyaning orol yoyi deb nomlangan tog 'jinslarida uchraydi lamprofir.[61]
Kimberlitlarni tor (1 dan 4 metrgacha) suv o'tkazgichlari va yonbag'irlarida va diametri taxminan 75 m dan 1,5 km gacha bo'lgan quvurlarda topish mumkin. Yangi tosh quyuq mavimsi yashildan yashil ranggacha kul rangga ega, ammo ta'sirlangandan keyin tezda jigarrang rangga aylanadi va parchalanadi.[68] Bu xaotik aralashgan kichik minerallar va tosh parchalari bilan gibrid tosh (Klaslar ) tarvuzlarning kattaligiga qadar. Ular aralashmasi ksenokristlar va ksenolitlar (pastki qobiq va mantiyadan ko'tarilgan minerallar va jinslar), er usti jinslari bo'laklari, o'zgartirilgan minerallar serpantin va portlash paytida kristallangan yangi minerallar. To'qimalarining chuqurligi bilan farq qiladi. Tarkibi bilan doimiylikni hosil qiladi karbonatitlar, ammo ikkinchisi uglerodning sof shaklda mavjud bo'lishi uchun juda ko'p kislorodga ega. Buning o'rniga, u mineralga yopilgan kaltsit (CaCO
3).[61]
Olmosli toshlarning uchtasida (kimberlit, lamproit va lamprofir) ma'lum minerallar yo'q (melilit va kalsilit ) olmos hosil bo'lishiga mos kelmaydigan. Kimberlitda, olivin katta va ko'zga tashlanadigan, lamproit esa Ti- ga ega.flogopit va lamprofir bor biotit va amfibol. Ularning barchasi oz miqdordagi eritmalardan tez otilib chiqadigan, boy bo'lgan magma turlaridan olingan uchuvchi va magniy oksidi va kamroq oksidlovchi kabi keng tarqalgan mantiya eriydi bazalt. Ushbu xususiyatlar eritmalar olmoslarni eritmasidan oldin yuzaga ko'tarib chiqishiga imkon beradi.[61]
Qidiruv

Kimberlit quvurlarini topish qiyin bo'lishi mumkin. Ular tezda ob-havoni (ta'sirlangandan keyin bir necha yil ichida) va atrofdagi toshlardan pastroq topografik relyefga ega bo'lishadi. Agar ular tashqi ko'rinishlarda ko'rinadigan bo'lsa, olmoslar hech qachon ko'rinmaydi, chunki ular juda kam. Har holda, kimberlitlar ko'pincha o'simlik, cho'kindi jinslar, tuproq yoki ko'llar bilan qoplanadi. Zamonaviy qidiruvlarda, geofizik usullar kabi aeromagnit tadqiqotlar, elektr qarshiligi va gravimetriya, o'rganish uchun istiqbolli hududlarni aniqlashga yordam bering. Bunga izotopik tanishish va geologik tarixni modellashtirish yordam beradi. Keyin topograflar hududga borib, kimberlit parchalarini qidirib, namunalarni to'plashlari kerak indikator minerallar. Ikkinchisida olmoslarning hosil bo'lish sharoitlarini aks ettiruvchi kompozitsiyalar mavjud, masalan, eritmaning haddan tashqari pasayishi yoki yuqori bosim eklogitlar. Biroq, indikatorli minerallar noto'g'ri bo'lishi mumkin; yaxshiroq yondashuv geotermobarometriya, bu erda minerallar tarkibi xuddi mantiya minerallari bilan muvozanatda bo'lganidek tahlil qilinadi.[61]
Kimberlitlarni topish qat'iylikni talab qiladi va faqat kichik bir qismda tijorat uchun foydali bo'lgan olmoslar mavjud. Taxminan 1980 yildan buyon yagona kashfiyot Kanadada bo'lgan. Mavjud konlarning ishlash muddati 25 yildan kam bo'lganligi sababli, kelajakda yangi olmoslar etishmasligi mumkin.[61]
Yoshlar
Olmoslar radioaktiv izotoplarning parchalanishi yordamida inkluziyalarni tahlil qilish bilan sanaladi. Elementar mo'llikka qarab, parchalanishga qarash mumkin rubidiydan stronsiyumgacha, samariydan neodimgacha, qo'rg'oshin uran, argon-40 dan argon-39gacha, yoki reniydan osmiygacha. Kimberlitlarda topilganlarning yoshi bir-biridan farq qiladi 1 dan 3,5 milliard yilgachava bir xil kimberlitda bir necha yosh bo'lishi mumkin, bu olmos hosil bo'lishining bir necha epizodlarini ko'rsatadi. Kimberlitlarning o'zlari ancha yoshroq. Ularning ko'pchiligining yoshi o'n milliondan 300 million yoshgacha, ammo ba'zi eski istisnolar mavjud (Argil, Premer va Wawa). Shunday qilib, kimberlitlar olmosdan mustaqil ravishda hosil bo'lgan va ularni faqat sirtga olib chiqish uchun xizmat qilgan.[19][61] Kimberlitlar, ular otib tashlagan kratonlarga qaraganda ancha yoshroq. Qadimgi kimberlitlarning etishmasligi sababi noma'lum, ammo mantiya kimyosi yoki tektonikasida ba'zi o'zgarishlar bo'lganligini ko'rsatadi. Insoniyat tarixida hech kimberlite otilmagan.[61]
Mantiyadan kelib chiqishi

Gavhari sifatli olmoslarning aksariyati litosferadagi 150–250 km chuqurlikdan olinadi. Bunday chuqurliklar kratonlar ostida joylashgan mantiya keellari, litosferaning eng qalin qismi. Ushbu mintaqalarda olmos paydo bo'lishiga imkon beradigan darajada yuqori bosim va harorat mavjud va ular konvektsiya qilinmaydi, shuning uchun olmoslar kimberlit otilishi ularni tanlamaguncha milliardlab yillar davomida saqlanishi mumkin.[61]
Mantiya keelidagi xost jinslari kiradi garsburgit va lerzolit, ikki turi peridotit. Jinslarining eng ustun jinsi yuqori mantiya, peridotit an magmatik tosh asosan minerallardan tashkil topgan olivin va piroksen; u past kremniy va yuqori magniy. Biroq, peridotitdagi olmoslar kamdan-kam uchraydigan sirtga omon qoladi.[61] Olmosni saqlaydigan yana bir keng tarqalgan manba bu eklogit, a metamorfik odatda hosil bo'lgan jins bazalt mantiya ichiga okean plitasi singib ketganida subduktsiya zonasi.[19]
Olmosning kichikroq qismi (150 ga yaqin o'rganilgan) 330–660 km chuqurlikdan kelib chiqadi, bu mintaqa o'tish zonasi. Ular eklogitda hosil bo'lgan, ammo qo'shilishlari bilan sayoz kelib chiqishi olmoslardan ajralib turadi majorit (shakli granat ortiqcha kremniy bilan). Olmoslarning o'xshash ulushi 660 dan 800 km gacha chuqurlikdagi pastki mantiyadan keladi.[19]
Olmos yuqori bosim va haroratda termodinamik barqaror, fazadan o'tish bilan grafit bosim oshishi bilan katta haroratlarda paydo bo'ladi. Shunday qilib, qit'alar ostida u 950 haroratda barqaror bo'ladi Selsiy daraja va 4,5 gigapaskal bosim, 150 chuqurlikka mos keladi kilometr yoki undan katta. Sovuqroq bo'lgan subduktsiya zonalarida u 800 ° S haroratda va 3,5 bosimda barqaror bo'ladi gigapaskallar. 240 km dan ortiq chuqurlikda temir-nikel metall fazalari mavjud va uglerod ularda yoki ular shaklida erigan bo'lishi mumkin karbidlar. Shunday qilib, ba'zi olmoslarning chuqurroq kelib chiqishi odatiy bo'lmagan o'sish muhitini aks ettirishi mumkin.[19][61]
2018 yilda muz fazasining ma'lum bo'lgan birinchi tabiiy namunalari chaqirildi Muz VII olmos namunalarida inklyuziya sifatida topilgan. Yuqori va pastki mantiya atrofida 400 dan 800 km gacha bo'lgan chuqurliklarda hosil bo'lgan qo'shimchalar va shu chuqurlikdagi suvga boy suyuqlik uchun dalillarni keltirib chiqaradi.[70][71]
Uglerod manbalari
Mantiya taxminan bir milliardga ega gigatonnalar uglerod (taqqoslash uchun atmosfera-okean tizimida taxminan 44000 gigatonn bor).[72] Uglerodning ikkitasi bor barqaror izotoplar, 12C va 13C, massa bo'yicha taxminan 99: 1 nisbatda.[61] Ushbu nisbat meteoritlarda keng diapazonga ega, bu uning Erning boshida ham juda xilma-xilligini anglatadi. Kabi sirt jarayonlari bilan ham o'zgartirilishi mumkin fotosintez. Fraktsiya odatda nisbati yordamida standart namunaga taqqoslanadi δ13C mingga qismlarda ifodalangan. Mantiyadagi bazaltlar, karbonatitlar va kimberlitlar kabi keng tarqalgan jinslar -8 dan -2 gacha bo'lgan nisbatlarga ega. Sirtda organik cho'kindilar o'rtacha -25, karbonatlar o'rtacha 0 ga teng.[19]
Turli manbalardan olingan olmos populyatsiyalari δ ning tarqalishiga ega13Aniq farq qiladigan C. Peridotitik olmoslar asosan mantiya doirasiga kiradi; eklogitik olmoslar -40 dan +3 gacha bo'lgan qiymatlarga ega, ammo tarqalish cho'qqisi mantiya oralig'ida. Ushbu o'zgaruvchanlik ularning ugleroddan hosil bo'lmaganligini anglatadi ibtidoiy (Yer paydo bo'lganidan beri mantiyada yashagan). Buning o'rniga, ular tektonik jarayonlarning natijasidir, garchi (olmoslarning yoshini hisobga olgan holda) hozirgi paytda amal qiladigan bir xil tektonik jarayonlar emas.[61]
Shakllanish va o'sish

Mantiyadagi olmoslar a orqali hosil bo'ladi metasomatik C-O-H-N-S suyuqligi yoki eritmasi minerallarni tog 'jinslarida eritib, ularni yangi minerallar bilan almashtiradigan jarayon. (C-O-H-N-S noaniq atamasi odatda ishlatiladi, chunki aniq tarkibi ma'lum emas.) Olmoslar bu suyuqlikdan oksidlangan uglerodni kamaytirish orqali hosil bo'ladi (masalan, CO2 yoki CO3) yoki kamaytirilgan fazaning oksidlanishi metan.[19]
Polarizatsiyalangan yorug'lik kabi problardan foydalanib, fotolüminesans va katodoluminesans, olmosda bir qator o'sish zonalarini aniqlash mumkin. Litosferadagi olmoslarning xarakterli namunasi lyuminesansiyada juda nozik tebranishlarga ega bo'lgan va kontsentrik zonalar qatorini o'z ichiga oladi va bu erda uglerod suyuqlik tomonidan so'rilib, keyin yana o'sib boradi. Litosfera ostidan hosil bo'lgan olmoslar ko'proq tartibsiz, deyarli polikristal to'qimalarga ega bo'lib, ular yuqori harorat va bosimni aks ettiradi, shuningdek olmosni konveksiya bilan tashishini aks ettiradi.[61]
Yer yuzasiga tashish

Geologik dalillar kimberlit magma soniyasiga 4-20 metr ko'tarilib, yuqoriga qarab yo'l yaratadigan modelni qo'llab-quvvatlaydi. gidravlik sinish toshning Bosim pasayganda, bug 'fazasi echadi magmadan kelib chiqadi va bu magma suyuqligini saqlashga yordam beradi. Er yuzida dastlabki portlash yoriqlar orqali yuqori tezlikda portlaydi (200 m / s (450 milya) dan yuqori). Keyinchalik, quyi bosimlarda jinslar yemirilib, quvur hosil qiladi va parchalangan tosh hosil qiladi (breccia ). Portlash susaygani sayin, bor piroklastik keyin metamorfizm va hidratsiya hosil bo'ladi serpentinitlar.[61]
Fazoda
Olmos yoqilgan bo'lsa-da Yer kamdan-kam uchraydi, ular kosmosda juda keng tarqalgan. Yilda meteoritlar, uglerodning uch foizga yaqin qismi nanodiamonds diametri bir necha nanometrga teng. Kosmik sovuqda etarlicha kichik olmoslar paydo bo'lishi mumkin, chunki ularning pastki qismi sirt energiyasi ularni grafitga qaraganda ancha barqaror qiladi. Ba'zi nanodiamondlarning izotopik imzolari, ular Quyosh tizimidan tashqarida yulduzlarda hosil bo'lganligini ko'rsatadi.[73]
Yuqori bosimli tajribalar shuni taxmin qiladiki, ko'p miqdordagi olmos kondanse qilinadi metan muz gigant sayyoralarida "olmos yomg'iriga" aylanadi Uran va Neptun.[74][75][76] Ba'zi bir ekstrasular sayyoralar deyarli olmosdan iborat bo'lishi mumkin.[77]
Olmos uglerodga boy yulduzlarda, xususan oq mitti. Kelib chiqishi uchun bitta nazariya karbonado, olmosning eng qiyin shakli shundaki, u oq mitti yoki supernova.[78][79] Yulduzlarda hosil bo'lgan olmoslar birinchi minerallar bo'lishi mumkin.[80]
Sanoat

Bugungi kunda olmoslarning eng taniqli ishlatilishi qimmatbaho toshlar kabi bezak, va qattiq materiallarni kesish uchun sanoat abraziv moddalar sifatida. Olmos toshlari va sanoat navlari olmoslari bozorlari olmoslarni turlicha baholaydi.

Qimmatbaho toshlar
The tarqalish ichiga oq nur spektral ranglar qimmatbaho olmoslarning asosiy gemologik xarakteristikasi. 20-asrda gemologiya bo'yicha mutaxassislar olmos va boshqa qimmatbaho toshlarni marvarid sifatida qadrlashi uchun eng muhim xususiyatlarga qarab graduslash usullarini ishlab chiqdilar. Norasmiy sifatida tanilgan to'rtta xususiyat to'rtta Cs, hozirda odatda olmosning asosiy tavsiflovchilari sifatida ishlatiladi: bu ularning massasi karat (karat 0,2 ga teng gramm), kesilgan (kesmaning sifati mos ravishda baholanadi nisbatlar, simmetriya va jilo ), rang (oq yoki rangsiz rangga qanchalik yaqin; chiroyli olmoslar uchun uning rangi qanchalik qizg'in) va aniqlik (bu qanchalik bepul qo'shimchalar ). Katta, beg'ubor olmos a nomi bilan tanilgan paragon.[81]
Qimmatbaho olmoslarning katta savdosi mavjud. Garchi qimmatbaho olmoslarning ko'pi yangi sayqallangan holda sotilsa-da, sayqallangan olmoslarni qayta sotish bo'yicha yaxshi yo'lga qo'yilgan bozor (masalan, garov garovi, kim oshdi savdosi, zargarlik buyumlari do'konlari, diamantaires, bouses va boshqalar) mavjud. Olmos savdosi sifatidagi olmos savdosining o'ziga xos xususiyati bu uning ajoyib kontsentratsiyasi: ulgurji savdo va olmosni kesish faqat bir nechta joylarda cheklangan; 2003 yilda dunyodagi olmoslarning 92% kesilgan va silliqlangan Surat, Hindiston.[82] Olmosni kesish va savdoning boshqa muhim markazlari Antverpen olmos tumani yilda Belgiya, qaerda Xalqaro gemologik institut Londonda joylashgan Olmos tumani Nyu-York shahrida Olmos birjasi tumani yilda Tel-Aviv va Amsterdam. Olmos konlarining geologik tabiati: bir nechta yirik birlamchi kimberlit-quvur konlari bozor ulushining muhim qismlarini (masalan, Jvaneng koni Botsvanada, bu yiliga 12 500 000 dan 15 000 000 karatgacha (2500 dan 3000 kg gacha) olmos ishlab chiqaradigan yagona yirik kon.[83]). Secondary alluvial diamond deposits, on the other hand, tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled.[84] This makes Antwerp a de facto "world diamond capital".[85] Antverpen shahri ham mezbonlarni qabul qiladi Antwerpsche Diamantkring, qo'pol olmoslarga bag'ishlangan birinchi va eng katta olmos birjasi bo'lish uchun 1929 yilda yaratilgan.[86] Another important diamond center is Nyu-York shahri, where almost 80% of the world's diamonds are sold, including auction sales.[84]
The De Beers company, as the world's largest diamond mining company, holds a dominant position in the industry, and has done so since soon after its founding in 1888 by the British businessman Sesil Rods. De Beers is currently the world's largest operator of diamond production facilities (mines) and tarqatish kanallari for gem-quality diamonds. The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines. De Beers and its subsidiaries own mines that produce some 40% of annual world diamond production. For most of the 20th century over 80% of the world's rough diamonds passed through De Beers,[87] but by 2001–2009 the figure had decreased to around 45%,[88] and by 2013 the company's market share had further decreased to around 38% in value terms and even less by volume.[89] De Beers sold off the vast majority of its diamond stockpile in the late 1990s – early 2000s[90] and the remainder largely represents working stock (diamonds that are being sorted before sale).[91] This was well documented in the press[92] but remains little known to the general public.
As a part of reducing its influence, De Beers withdrew from purchasing diamonds on the open market in 1999 and ceased, at the end of 2008, purchasing Russian diamonds mined by the largest Russian diamond company Alrosa.[93] As of January 2011, De Beers states that it only sells diamonds from the following four countries: Botswana, Namibia, South Africa and Canada.[94] Alrosa had to suspend their sales in October 2008 due to the global energy crisis,[95] but the company reported that it had resumed selling rough diamonds on the open market by October 2009.[96] Apart from Alrosa, other important diamond mining companies include BHP Billiton, which is the world's largest mining company;[97] Rio Tinto guruhi, egasi Argil (100%), Diavik (60%), and Murova (78%) diamond mines;[98] va Petra Diamonds, the owner of several major diamond mines in Africa.

Further down the supply chain, members of The Jahon brilliantlar federatsiyasi (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia.[30] In 2000, the WFDB and The International Diamond Manufacturers Association established the Jahon olmos kengashi to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the Jahon olmos kongressi every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives).[99] The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide.[99] Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Tailand, Namibia and Botswana.[99] Cutting centers with lower cost of labor, notably Surat in Gujarat, Hindiston, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.[84]
Diamonds prepared as gemstones are sold on diamond exchanges called birjalar. There are 28 registered diamond bourses in the world.[100] Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.[101]
Kesish

Mined rough diamonds are converted into gems through a multi-step process called "cutting". Diamonds are extremely hard, but also brittle and can be split up by a single blow. Therefore, diamond cutting is traditionally considered as a delicate procedure requiring skills, scientific knowledge, tools and experience. Its final goal is to produce a faceted jewel where the specific angles between the facets would optimize the diamond luster, that is dispersion of white light, whereas the number and area of facets would determine the weight of the final product. The weight reduction upon cutting is significant and can be of the order of 50%.[102] Several possible shapes are considered, but the final decision is often determined not only by scientific, but also practical considerations. For example, the diamond might be intended for display or for wear, in a ring or a necklace, singled or surrounded by other gems of certain color and shape.[103] Some of them may be considered as classical, such as dumaloq, nok, marquise, tuxumsimon, qalblar va o'qlar diamonds, etc. Some of them are special, produced by certain companies, for example, Feniks, Yostiq, Sole Mio diamonds, etc.[104]
The most time-consuming part of the cutting is the preliminary analysis of the rough stone. It needs to address a large number of issues, bears much responsibility, and therefore can last years in case of unique diamonds. The following issues are considered:
- The hardness of diamond and its ability to cleave strongly depend on the crystal orientation. Therefore, the crystallographic structure of the diamond to be cut is analyzed using Rentgen difraksiyasi to choose the optimal cutting directions.
- Most diamonds contain visible non-diamond inclusions and crystal flaws. The cutter has to decide which flaws are to be removed by the cutting and which could be kept.
- The diamond can be split by a single, well calculated blow of a hammer to a pointed tool, which is quick, but risky. Alternatively, it can be cut with a olmos arra, which is a more reliable but tedious procedure.[103][105]
After initial cutting, the diamond is shaped in numerous stages of polishing. Unlike cutting, which is a responsible but quick operation, polishing removes material by gradual erosion and is extremely time consuming. The associated technique is well developed; it is considered as a routine and can be performed by technicians.[106] After polishing, the diamond is reexamined for possible flaws, either remaining or induced by the process. Those flaws are concealed through various diamond enhancement techniques, such as repolishing, crack filling, or clever arrangement of the stone in the jewelry. Remaining non-diamond inclusions are removed through laser drilling and filling of the voids produced.[57]
Marketing

Marketing has significantly affected the image of diamond as a valuable commodity.
N. V. Ayer va O'g'il, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market. And the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included mahsulotni joylashtirish, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well,[107] but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to 2nd place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold.[108] The campaign lasted for decades but was effectively discontinued by early 2011. De Beers still advertises diamonds, but the advertising now mostly promotes its own brands, or licensed product lines, rather than completely "generic" diamond products.[108] The campaign was perhaps best captured by the slogan "a diamond is forever ".[109] This slogan is now being used by De Beers Diamond Jewelers,[110] a jewelry firm which is a 50%/50% joint venture between the De Beers mining company and LVMH, the luxury goods conglomerate.
Brown-colored diamonds constituted a significant part of the diamond production, and were predominantly used for industrial purposes. They were seen as worthless for jewelry (not even being assessed on the olmos rangi o'lchov). After the development of Argyle diamond mine in Australia in 1986, and marketing, brown diamonds have become acceptable gems.[111][112] The change was mostly due to the numbers: the Argyle mine, with its 35,000,000 carats (7,000 kg) of diamonds per year, makes about one-third of global production of natural diamonds;[113] 80% of Argyle diamonds are brown.[114]
Industrial-grade diamonds



Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. 80% of mined diamonds (equal to about 135,000,000 carats (27,000 kg) annually) are unsuitable for use as gemstones and are used industrially.[115] In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 570,000,000 carats (114,000 kg) of synthetic diamond is produced annually for industrial use (in 2004; in 2014 it is 4,500,000,000 carats (900,000 kg), 90% of which is produced in China). Approximately 90% of diamond grinding grit is currently of synthetic origin.[116]
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some lower-grade stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort.[117]
Industrial use of diamonds has historically been associated with their hardness, which makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this property include diamond-tipped matkap uchlari and saws, and the use of diamond powder as an abraziv. Less expensive industrial-grade diamonds, known as bort, with more flaws and poorer color than gems, are used for such purposes.[118] Diamond is not suitable for machining qora qotishmalar at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.[119]
Specialized applications include use in laboratories as containment for high-pressure experiments (qarang olmos anvil hujayrasi ), high-performance rulmanlar, and limited use in specialized derazalar.[117] With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. Yuqori issiqlik o'tkazuvchanligi of diamond makes it suitable as a kuler for integrated circuits in elektronika.[120]
Konchilik
Approximately 130,000,000 carats (26,000 kg) of diamonds are mined annually, with a total value of nearly US$9 billion, and about 100,000 kg (220,000 lb) are synthesized annually.[121]
Roughly 49% of diamonds originate from Markaziy va Janubiy Afrika, although significant sources of the mineral have been discovered in Kanada, Hindiston, Rossiya, Braziliya va Avstraliya.[116] They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as concerns over the sale of qon olmoslari yoki ziddiyatli olmoslar by African harbiylashtirilgan guruhlar.[122] The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world.
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care is required not to destroy larger diamonds, and then sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of Rentgen lyuminestsentsiyasi, after which the final sorting steps are done by hand. Before the use of X-nurlari became commonplace,[102] the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.[30]

Historically, diamonds were found only in allyuvial yotqiziqlar yilda Guntur va Krishna tumani ning Krishna daryosi delta Janubiy Hindiston.[123] India led the world in diamond production from the time of their discovery in approximately the 9th century BC[124][125] to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.[124] Currently, one of the most prominent Indian mines is located at Panna.[126]
Diamond extraction from primary deposits (kimberlites and lamproites) started in the 1870s after the discovery of the Olmos maydonlari Janubiy Afrikada.[127]Production has increased over time and now an accumulated total of 4,500,000,000 carats (900,000 kg) have been mined since that date.[128] Twenty percent of that amount has been mined in the last five years, and during the last 10 years, nine new mines have started production; four more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.[128]
In the U.S., diamonds have been found in Arkanzas, Kolorado, Nyu-Meksiko, Wyoming, and Montana.[129][130] In 2004, the discovery of a microscopic diamond in the U.S. led to the January 2008 bulk-sampling of kimberlit quvurlar in a remote part of Montana. The Diamonds shtatidagi parkning krateri yilda Arkanzas is open to the public, and is the only mine in the world where members of the public can dig for diamonds.[130]
Today, most commercially viable diamond deposits are in Russia (mostly in Saxa Respublikasi, masalan Mir pipe va Udachnaya trubkasi ), Botsvana, Avstraliya (Shimoliy va G'arbiy Avstraliya ) va Kongo Demokratik Respublikasi.[131]In 2005, Russia produced almost one-fifth of the global diamond output, according to the Britaniya geologik xizmati. Australia boasts the richest diamantiferous pipe, with production from the Argyle diamond mine reaching peak levels of 42 metric tons per year in the 1990s.[129][132]There are also commercial deposits being actively mined in the Shimoli-g'arbiy hududlar of Canada and Brazil.[116]Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
Siyosiy masalalar
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of olmos konlari, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as ziddiyatli olmoslar yoki qon olmoslari.[122]
In response to public concerns that their diamond purchases were contributing to war and inson huquqlarining buzilishi yilda markaziy va g'arbiy Afrika, Birlashgan Millatlar, the diamond industry and diamond-trading nations introduced the Kimberley jarayoni 2002 yilda.[133] The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. This is done by requiring diamond-producing countries to provide proof that the money they make from selling the diamonds is not used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, some still find their way in. According to the International Diamond Manufacturers Association, conflict diamonds constitute 2–3% of all diamonds traded.[134] Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered "clean".[133]
The Canadian Government has set up a body known as the Canadian Diamond Code of Conduct[135] to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the "conflict free" label of Canadian diamonds.[136]
Synthetics, simulants, and enhancements
Synthetics

Synthetic diamonds are diamonds manufactured in a laboratory, as opposed to diamonds mined from the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.[60] It is possible to make colorless synthetic gemstones that, on a molecular level, are identical to natural stones and so visually similar that only a gemologist with special equipment can tell the difference.[137]
The majority of commercially available synthetic diamonds are yellow and are produced by so-called high-pressure high-temperature (HPHT ) jarayonlar.[138] The yellow color is caused by azot impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of bor yoki dan nurlanish after synthesis.[139]

Another popular method of growing synthetic diamond is kimyoviy bug 'cho'kmasi (KVH). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically 1 dan 99 gacha metan ga vodorod ) into a chamber and splitting them to chemically active radikallar a plazma ignited by mikroto'lqinli pechlar, issiq filaman, yoy oqimi, payvandlash mash'alasi yoki lazer.[140] This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).[121]
As of 2010, nearly all 5,000 million carats (1,000 tonnes) of synthetic diamonds produced per year are for industrial use. Around 50% of the 133 million carats of natural diamonds mined per year end up in industrial use.[137][141] Mining companies' expenses average 40 to 60 US dollars per carat for natural colorless diamonds, while synthetic manufacturers' expenses average $2,500 per carat for synthetic, gem-quality colorless diamonds.[137]:79 However, a purchaser is more likely to encounter a synthetic when looking for a fancy-colored diamond because nearly all synthetic diamonds are fancy-colored, while only 0.01% of natural diamonds are.[142]
Simulyantlar

A diamond simulant is a non-diamond material that is used to simulate the appearance of a diamond, and may be referred to as diamante. Kubik tsirkoniya eng keng tarqalgan. The gemstone moissanit (silicon carbide) can be treated as a diamond simulant, though more costly to produce than cubic zirconia. Both are produced synthetically.[143]
Yaxshilashlar
Diamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.[144]
Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more "diamond-like" appearance. One such substance is olmosga o'xshash uglerod —an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. Kabi usullar Raman spektroskopiyasi should easily identify such a treatment.[145]
Identifikatsiya
Early diamond identification tests included a scratch test relying on the superior hardness of diamond. This test is destructive, as a diamond can scratch another diamond, and is rarely used nowadays. Instead, diamond identification relies on its superior thermal conductivity. Electronic thermal probes are widely used in the gemological centers to separate diamonds from their imitations. These probes consist of a pair of battery-powered termistorlar mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about two to three seconds.[146]
Whereas the thermal probe can separate diamonds from most of their simulants, distinguishing between various types of diamond, for example synthetic or natural, irradiated or non-irradiated, etc., requires more advanced, optical techniques. Those techniques are also used for some diamonds simulants, such as silicon carbide, which pass the thermal conductivity test. Optical techniques can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds.[147] "Perfect" crystals (at the atomic lattice level) have never been found, so both natural and synthetic diamonds always possess characteristic imperfections, arising from the circumstances of their crystal growth, that allow them to be distinguished from each other.[148]
Laboratories use techniques such as spectroscopy, microscopy and luminescence under shortwave ultraviolet light to determine a diamond's origin.[147] They also use specially made instruments to aid them in the identification process. Two screening instruments are the DiamondSure va DiamondView, ikkalasi tomonidan ishlab chiqarilgan DTC and marketed by the GIA.[149]
Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D-J colored diamonds can be screened through the Swiss Gemmological Institute "s[150] Diamond Spotter. Stones in the D-Z color range can be examined through the DiamondSure UV/visible spectrometer, a tool developed by De Beers.[148] Similarly, natural diamonds usually have minor imperfections and flaws, such as inclusions of foreign material, that are not seen in synthetic diamonds.
Screening devices based on diamond type detection can be used to make a distinction between diamonds that are certainly natural and diamonds that are potentially synthetic. Those potentially synthetic diamonds require more investigation in a specialized lab. Examples of commercial screening devices are D-Screen (WTOCD / HRD Antwerp), Alpha Diamond Analyzer (Bruker / HRD Antwerp) and D-Secure (DRC Techno).
O'g'irlik
Occasionally, large thefts of diamonds take place. In February 2013 armed robbers carried out a raid at Brussels Airport and escaped with gems estimated to be worth US$50M (£32M; €37M). The gang broke through a perimeter fence and raided the cargo hold of a Swiss-bound plane. The gang have since been arrested and large amounts of cash and diamonds recovered.[151]
The identification of stolen diamonds presents a set of difficult problems. Rough diamonds will have a distinctive shape depending on whether their source is a mine or from an alluvial environment such as a beach or river—alluvial diamonds have smoother surfaces than those that have been mined. Determining the provenance of cut and polished stones is much more complex.
The Kimberley jarayoni was developed to monitor the trade in rough diamonds and prevent their being used to fund violence. Before exporting, rough diamonds are certificated by the government of the country of origin. Some countries, such as Venezuela, are not party to the agreement. The Kimberley Process does not apply to local sales of rough diamonds within a country.
Diamonds may be etched by laser with marks invisible to the naked eye. Lazare Kaplan, a US-based company, developed this method. However, whatever is marked on a diamond can readily be removed.[152][153]
Etymology, earliest use and composition discovery
Ism olmos is derived from the ancient Greek ἀδάmáb (adámas), "proper", "unalterable", "unbreakable", "untamed", from ἀ- (a-), "un-" + gámάω (damáō), "I overpower", "I tame".[154] Diamonds are thought to have been first recognized and mined in Hindiston, qaerda muhim allyuvial yotqiziqlar of the stone could be found many centuries ago along the rivers Penner, Krishna va Godavari. Diamonds have been known in India for at least 3,000 years but most likely 6,000 yil.[124]
Diamonds have been treasured as gemstones since their use as religious icons yilda qadimgi Hindiston. Their usage in engraving tools also dates to early insoniyat tarixi.[155][156] The popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.[109]
In 1772, the French scientist Antuan Lavuazye used a lens to concentrate the rays of the sun on a diamond in an atmosphere of kislorod, and showed that the only product of the combustion was karbonat angidrid, proving that diamond is composed of carbon.[157] Later in 1797, the English chemist Smitson Tennant repeated and expanded that experiment.[158] By demonstrating that burning diamond and graphite releases the same amount of gas, he established the chemical equivalence of these substances.[58]
Shuningdek qarang
- Chuqur uglerod aylanishi
- Diamondoid
- Olmoslar ro'yxati
- Minerallarning ro'yxati
- Superhard material
- Extraterrestrial diamonds
Adabiyotlar
- ^ a b "Olmos". Mindat. Olingan 7 iyul, 2009.
- ^ "Olmos". WebMineral. Olingan 7 iyul, 2009.
- ^ Delhaes, Pierre (2000). "Polymorphism of carbon". In Delhaes, Pierre (ed.). Graphite and precursors. Gordon va buzish. 1-24 betlar. ISBN 9789056992286.
- ^ Pierson, Hugh O. (2012). Handbook of carbon, graphite, diamond, and fullerenes : properties, processing, and applications. Noyes nashrlari. 40-41 betlar. ISBN 9780815517399.
- ^ Angus, J. C. (1997). "Structure and thermochemistry of diamond". In Paoletti, A.; Tucciarone, A. (eds.). The physics of diamond. IOS Press. 9-30 betlar. ISBN 9781614992202.
- ^ a b Rock, Peter A. (1983). Kimyoviy termodinamika. Universitet ilmiy kitoblari. 257-260 betlar. ISBN 9781891389320.
- ^ Gray, Theodore (October 8, 2009). "Gone in a Flash". Ommabop fan. Olingan 31 oktyabr, 2018.
- ^ Chen, Yiqing; Zhang, Liangchi (2013). Polishing of diamond materials : mechanisms, modeling and implementation. Springer Science & Business Media. pp.1 –2. ISBN 9781849964081.
- ^ a b Bundy, P.; Bassett, W. A.; Weathers, M. S.; Hemley, R. J.; Mao, H. K.; Goncharov, A. F. (1996). "The pressure-temperature phase and transformation diagram for carbon; updated through 1994". Uglerod. 34 (2): 141–153. doi:10.1016/0008-6223(96)00170-4.
- ^ Vang, C. X .; Yang, G. W. (2012). "Thermodynamic and kinetic approaches of diamond and related nanomaterials formed by laser ablation in liquid". In Yang, Guowei (ed.). Laser ablation in liquids : principles and applications in the preparation of nanomaterials. Pan Stanford Pub. 164-165 betlar. ISBN 9789814241526.
- ^ Wang, Xiaofei; Scandolo, Sandro; Car, Roberto (October 25, 2005). "Carbon Phase Diagram from Ab Initio Molecular Dynamics". Jismoniy tekshiruv xatlari. 95 (18): 185701. Bibcode:2005PhRvL..95r5701W. doi:10.1103/PhysRevLett.95.185701. PMID 16383918.
- ^ Correa, A. A.; Bonev, S. A.; Galli, G. (January 23, 2006). "Carbon under extreme conditions: Phase boundaries and electronic properties from first-principles theory". Milliy fanlar akademiyasi materiallari. 103 (5): 1204–1208. Bibcode:2006PNAS..103.1204C. doi:10.1073/pnas.0510489103. PMC 1345714. PMID 16432191.
- ^ Eric Bland (January 15, 2010). "Diamond oceans possible on Uranus, Neptune". Discovery News. Olingan 16 yanvar, 2010.
- ^ Silvera, Ishoq (2010). "Olmos: bosim ostida eritilgan". Tabiat fizikasi. 6 (1): 9–10. Bibcode:2010 yil NatPh ... 6 .... 9S. doi:10.1038 / nphys1491.
- ^ Rajendran, V. (2004). Materialshunoslik. Tata McGraw-Hill Pub. p. 2.16. ISBN 9780070583696.
- ^ a b Eshkroft, Nil V.; Mermin, N. Devid (1976). Qattiq jismlar fizikasi. Xolt, Raynxart va Uinston. p.76. ISBN 978-0030839931.
- ^ Bandosz, Teresa J.; Biggs, Mark J.; Gubbins, Keith E.; Hattori, Y.; Iiyama, T.; Kaneko, Tatsumi; Pikunic, Jorge; Thomson, Kendall (2003). "Molecular models of porous carbons". In Radovic, Ljubisa R. (ed.). Chemistry and physics of carbon. 28. Marsel Dekker. 46-47 betlar. ISBN 9780824709877.
- ^ Webster, R.; Read, P.G. (2000). Gems: Their sources, descriptions and identification (5-nashr). Buyuk Britaniya: Butterworth-Heinemann. p. 17. ISBN 978-0-7506-1674-4.
- ^ a b v d e f g h men j Cartigny, Per; Palot, Médéric; Thomassot, Emilie; Harris, Jeff W. (May 30, 2014). "Diamond Formation: A Stable Isotope Perspective". Yer va sayyora fanlari bo'yicha yillik sharh. 42 (1): 699–732. Bibcode:2014AREPS..42..699C. doi:10.1146/annurev-earth-042711-105259.
- ^ Fukura, Satoshi; Nakagawa, Tatsuo; Kagi, Hiroyuki (November 2005). "High spatial resolution photoluminescence and Raman spectroscopic measurements of a natural polycrystalline diamond, carbonado". Olmos va tegishli materiallar. 14 (11–12): 1950–1954. Bibcode:2005DRM....14.1950F. doi:10.1016/j.diamond.2005.08.046.
- ^ Garai, J.; Haggerty, S.E.; Rekhi, S.; Chance, M. (2006). "Infrared Absorption Investigations Confirm the Extraterrestrial Origin of Carbonado Diamonds". Astrofizika jurnali. 653 (2): L153-L156. arXiv:physics/0608014. Bibcode:2006ApJ...653L.153G. doi:10.1086/510451. S2CID 59405368.
- ^ "Diamonds from Outer Space: Geologists Discover Origin of Earth's Mysterious Black Diamonds". Milliy Ilmiy Jamg'arma. 2007 yil 8-yanvar. Olingan 28 oktyabr, 2007.
- ^ "Diamonds Are Indestructible, Right?". Dominion Jewelers. 2015-12-16. Olingan 2020-10-31.
- ^ M. Seal, "Olmosning aşınması", Qirollik jamiyati materiallari A 248: 1254 (1958 yil 25-noyabr) doi:10.1098 / rspa.1958.0250
- ^ Garold D. Vayler, "Yozuvlar va uslublarning kiyinishi va parvarishi", 1954, qisqartirilgan matn
- ^ Neves, A. J.; Nazaré, M. H. (2001). Properties, Growth and Applications of Diamond. Muhandislik va texnologiya instituti. pp. 142–147. ISBN 978-0-85296-785-0.
- ^ Boser, U. (2008). "Diamonds on Demand". Smithsonian. 39 (3): 52–59.
- ^ Li J.; Novikov, N. V. (2005). Innovative superhard materials and sustainable coatings for advanced manufacturing. Springer. p. 102. ISBN 978-0-8493-3512-9.
- ^ Marinescu, I. D.; Tönshoff, H. K.; Inasaki, I. (2000). Handbook of ceramic grinding and polishing. Uilyam Endryu. p. 21. ISBN 978-0-8155-1424-4.
- ^ a b v d e f Harlow, G.E. (1998). The nature of diamonds. Kembrij universiteti matbuoti. p. 223, 230–249. ISBN 978-0-521-62935-5.
- ^ Eremets, Mikhail I.; Trojan, Ivan A.; Gwaze, Patience; Huth, Joachim; Boehler, Reinhard; Blank, Vladimir D. (October 3, 2005). "The strength of diamond". Amaliy fizika xatlari. 87 (14): 141902. doi:10.1063/1.2061853.
- ^ a b Dubrovinsky, Leonid; Dubrovinskaya, Natalya; Prakapenka, Vitali B; Abakumov, Artem M (October 23, 2012). "Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar". Tabiat aloqalari. 3 (1): 1163. Bibcode:2012NatCo...3E1163D. doi:10.1038/ncomms2160. PMC 3493652. PMID 23093199.
- ^ Improved diamond anvil cell allows higher pressures Fizika olami 2012 yil noyabr.
- ^ "Improved diamond-anvil cell allows higher pressures than ever before – Physics World". Fizika olami. 2012 yil 2-noyabr. Olingan 1-noyabr, 2018.
- ^ Banerjee, Amit; va boshq. (2018 yil 20-aprel). "Ultralarge elastic deformation of nanoscale diamond". Ilm-fan. 360 (6386): 300–302. doi:10.1126/science.aar4165. PMID 29674589.
- ^ LLorca, Javier (April 20, 2018). "On the quest for the strongest materials". Ilm-fan. 360 (6386): 264–265. doi:10.1126/science.aat5211. PMID 29674578. S2CID 4986592.
- ^ Collins, A. T. (1993). "Yarimo'tkazgichli olmosning optik va elektron xususiyatlari". Qirollik jamiyatining falsafiy operatsiyalari A. 342 (1664): 233–244. Bibcode:1993RSPTA.342..233C. doi:10.1098 / rsta.1993.0017. S2CID 202574625.
- ^ Landstrass, M. I.; Ravi, K. V. (1989). "Resistivity of chemical vapor deposited diamond films". Amaliy fizika xatlari. 55 (10): 975–977. Bibcode:1989ApPhL..55..975L. doi:10.1063/1.101694.
- ^ Chjan, V.; Ristein, J.; Ley, L. (2008). "Hydrogen-terminated diamond electrodes. II. Redox activity". Jismoniy sharh E. 78 (4): 041603. Bibcode:2008PhRvE..78d1603Z. doi:10.1103/PhysRevE.78.041603. PMID 18999435.
- ^ Zhe, Shi (5 October 2020). "Metallization of Diamond". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari.
- ^ Wissner-Gross, A. D.; Kaxiras, E. (2007). "Diamond stabilization of ice multilayers at human body temperature" (PDF). Jismoniy sharh E. 76 (2): 020501. Bibcode:2007PhRvE..76b0501W. doi:10.1103/physreve.76.020501. PMID 17929997.
- ^ Fujimoto, A.; Yamada, Y .; Koinuma, M.; Sato, S. (2016). "Origins of sp3C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials". Analitik kimyo. 88 (12): 6110–4. doi:10.1021/acs.analchem.6b01327. PMID 27264720.
- ^ Bauer, Max (2012). Precious Stones, Volume 1. Dover nashrlari. 115–117 betlar. ISBN 9780486151250.
- ^ "Diamond Care and Cleaning Guide". Amerikaning gemologik instituti. Olingan 1 avgust 2019.
- ^ Jones, Carl (27 August 2016). "Diamonds are Flammable! How to Safeguard Your Jewelry". DMIA. Olingan 1 avgust 2019.
- ^ Baird, Christopher S. "Can you light diamond on fire?". Science Questions with Surprising Answers. Olingan 1 avgust 2019.
- ^ Lederle, Feliks; Koch, Yannis; Xyubner, Eike G. (21 fevral, 2019). "Rangli uchqunlar". Evropa noorganik kimyo jurnali. 2019 (7): 928–937. doi:10.1002 / ejic.201801300.
- ^ Kollinz, A. T .; Kanda, Xisao; Isoya, J .; Ammerlaan, C. A. J.; Van Uik, J. A. (1998). "Nikel erituvchi katalizatoridan yetishtirilgan yuqori bosimli olmosda optik yutilish va EPR o'rtasidagi o'zaro bog'liqlik". Olmos va tegishli materiallar. 7 (2–5): 333–338. Bibcode:1998DRM ..... 7..333C. doi:10.1016 / S0925-9635 (97) 00270-7.
- ^ Zaitsev, A. M. (2000). "Olmosdagi nopoklik bilan bog'liq optik markazlarning vibronik spektrlari". Jismoniy sharh B. 61 (19): 12909–12922. Bibcode:2000PhRvB..6112909Z. doi:10.1103 / PhysRevB.61.12909.
- ^ Walker, J. (1979). "Olmosdagi optik yutish va lyuminestsentsiya" (PDF). Fizikada taraqqiyot haqida hisobotlar. 42 (10): 1605–1659. Bibcode:1979RPPh ... 42.1605W. CiteSeerX 10.1.1.467.443. doi:10.1088/0034-4885/42/10/001.
- ^ Xounsom, L. S .; Jons, R .; Shou, M. J .; Briddon, P. R .; Öberg, S .; Briddon, P .; Öberg, S. (2006). "Olmosdagi jigarrang rangning kelib chiqishi". Jismoniy sharh B. 73 (12): 125203. Bibcode:2006PhRvB..73l5203H. doi:10.1103 / PhysRevB.73.125203.
- ^ Wise, R. W. (2001). Qimmatbaho toshlar savdosi sirlari, Qimmatbaho toshlar uchun biluvchilar uchun qo'llanma. Brunsvik uyi matbuoti. 223-224 betlar. ISBN 978-0-9728223-8-1.
- ^ Khan, Urmee (2008 yil 10-dekabr). "Ispaniya qiroliga tegishli ko'k-kulrang olmos rekord darajadagi 16,3 ga sotildi GBP". Daily Telegraph. London. Olingan 31 mart, 2010.
- ^ Nebehay, S. (2009 yil 12-may). "Noyob ko'k olmos rekord darajada 9,5 million dollarga sotiladi". Reuters. Olingan 13 may, 2009.
- ^ Pomfret, Jeyms (2009 yil 1-dekabr). "Jonli pushti olmos rekord darajada 10,8 million dollarga sotildi". Reuters.
- ^ Vey, L .; Kuo, P. K .; Tomas, R.L .; Entoni, T .; Banxolzer, V. (1993). "Izotopik modifikatsiyalangan yagona kristall olmosning issiqlik o'tkazuvchanligi". Jismoniy tekshiruv xatlari. 70 (24): 3764–3767. Bibcode:1993PhRvL..70.3764W. doi:10.1103 / PhysRevLett.70.3764. PMID 10053956.
- ^ a b O'qing, P. G. (2005). Gemmologiya. Butterworth-Heinemann. 165–166 betlar. ISBN 978-0-7506-6449-3.
- ^ a b Hazen, R. M. (1999). Olmos ishlab chiqaruvchilar. Kembrij universiteti matbuoti. 7-10 betlar. ISBN 978-0-521-65474-6.
- ^ O'Donoghue, M. (1997). Sintetik, taqlid va ishlov berilgan qimmatbaho toshlar. Gulf Professional Publishing. 34-37 betlar. ISBN 978-0-7506-3173-0.
- ^ a b v Erlich, Edvard I.; Hausel, V. Dan (2002). Olmos konlari: kelib chiqishi, qidiruvi va kashfiyot tarixi. Littleton, CO: Konchilik, metallurgiya va razvedka jamiyati. ISBN 978-0-87335-213-0.
- ^ a b v d e f g h men j k l m n o p q r Shiri, Stiven B.; Shigli, Jeyms E. (2013 yil 1-dekabr). "Olmoslar geologiyasini tushunishda so'nggi yutuqlar". Toshlar va gemologiya. 49 (4): 188–222. doi:10.5741 / GEMS.49.4.188.
- ^ Karlson, RW (2005). Mantiya va yadro. Elsevier. p. 248. ISBN 978-0-08-044848-0.
- ^ Deutsch, Aleksandr; Masaitis, V.L .; Langenhorst, F.; Grieve, R.A.F. (2000). "Popigai, Sibir - yaxshi saqlanib qolgan ulkan zarba tuzilishi, milliy xazina va dunyo geologik merosi" (PDF). Qismlar. 23 (1): 3–12. doi:10.18814 / epiiugs / 2000 / v23i1 / 002. Arxivlandi asl nusxasi (PDF) 2012 yil 21 oktyabrda. Olingan 16 iyun, 2008.
- ^ King, Hobart (2012). "Olmos qanday hosil bo'ladi? Ular ko'mirdan hosil bo'lmaydi!". Geologiya va Yer haqidagi yangiliklar va ma'lumotlar. geologiya.com. Arxivlandi asl nusxasidan 2013 yil 30 oktyabrda. Olingan 29 iyun, 2012.
- ^ Pak-Xarvi, Ameliya (2013 yil 31 oktyabr). "10 ta keng tarqalgan ilmiy noto'g'ri tushunchalar". Christian Science Monitor. Olingan 30 avgust, 2017.
- ^ Pohl, Valter L. (2011). Iqtisodiy geologiya: printsiplari va amaliyoti. John Wiley & Sons. ISBN 9781444394863.
- ^ Allaby, Maykl (2013). "mobil kamar". Geologiya va er haqidagi lug'at (4-nashr). Oksford: Oksford universiteti matbuoti. ISBN 9780191744334.
- ^ Kjarsgaard, B. A. (2007). "Kimberlite quvurlari modellari: razvedka uchun ahamiyati" (PDF). Milkereitda B. (tahr.) Exploration 07 materiallari: foydali qazilmalarni qidirish bo'yicha beshinchi o'n yillik xalqaro konferentsiya. O'n yillik foydali qazilmalarni qidirish bo'yicha konferentsiyalar, 2007. 667–677 betlar. Olingan 1 mart, 2018.
- ^ a b Deep Carbon Observatory (2019). Chuqur uglerod rasadxonasi: kashfiyotning o'n yilligi. Vashington, DC. doi:10.17863 / CAM.44064. Olingan 13 dekabr 2019.
- ^ Cartier, Kimberly (2018 yil 2-aprel). "Olmos aralashmalari mantiya ostidagi suvni ochib beradi". Eos. 99. doi:10.1029 / 2018EO095949.
- ^ Perkins, Sid (2018 yil 8 mart). "Suv cho'ntaklari Yer yuzasidan pastda yotishi mumkin". Ilm-fan.
- ^ Li, C-T. A .; Tszyan, X.; Dasgupta, R .; Torres, M. (2019). "Butun er yuzidagi uglerod velosipedini velosiped haydashni tushunish uchun asos". Orkutda, Bet N.; Daniel, Izabel; Dasgupta, Rajdeep (tahrir). Chuqur uglerod: o'tmishdan hozirgi kungacha. Kembrij universiteti matbuoti. 313-357 betlar. doi:10.1017/9781108677950.011. ISBN 9781108677950.
- ^ Tielens, A. G. G. M. (2013 yil 12-iyul). "Molekulyar koinot". Zamonaviy fizika sharhlari. 85 (3): 1021–1081. Bibcode:2013RvMP ... 85.1021T. doi:10.1103 / RevModPhys.85.1021.
- ^ Kerr, R. A. (1999 yil 1 oktyabr). "Neptun metanni olmoslarga ezishi mumkin". Ilm-fan. 286 (5437): 25a – 25. doi:10.1126 / science.286.5437.25a. PMID 10532884. S2CID 42814647.
- ^ Skandolo, Sandro; Janloz, Raymond (2003 yil noyabr-dekabr). "Sayyoralar markazlari: Laboratoriyalarda va kompyuterlarda zarba va siqilgan moddalar metallga aylanadi, olmos bilan yo'taladi va Yerning oq-issiq markazini ochib beradi". Amerikalik olim. 91 (6): 516–525. Bibcode:2003AmSci..91..516S. doi:10.1511/2003.38.905. JSTOR 27858301.
- ^ Kaplan, Sara (2017 yil 25-avgust). "Uran va Neptunga qattiq olmoslar yog'moqda". Vashington Post. Olingan 16 oktyabr, 2017.
- ^ Maks Plank nomidagi Radio Astronomiya Instituti (2011 yil 25 avgust). "Olmosdan yasalgan sayyora". Astronomiya jurnali. Olingan 25 sentyabr, 2017.
- ^ Xeni, P. J.; Vicenzi, E. P.; De, S. (2005). "G'alati olmoslar: karbonado va ramzitning sirli kelib chiqishi". Elementlar. 1 (2): 85–89. doi:10.2113 / gselements.1.2.85.
- ^ Shumilova, T.G .; Tkachev, S.N .; Isaenko, S.I .; Shevchuk, S.S .; Rappenglyuk, M.A .; Kazakov, V.A. (2016 yil aprel). "Laboratoriyada" olmosga o'xshash yulduz ". Olmosga o'xshash shisha". Uglerod. 100: 703–709. doi:10.1016 / j.karbon.2016.01.068.
- ^ Vey-Xaas, Mayya. "Hayot va toshlar er yuzida birgalikda rivojlangan bo'lishi mumkin". Smithsonian. Olingan 26 sentyabr, 2017.
- ^ Gessen, R. V. (2007). Tarix orqali zargarlik buyumlari ishlab chiqarish. Greenwood Publishing Group. p. 42. ISBN 978-0-313-33507-5.
- ^ Adiga, A. (2004 yil 12 aprel). "Noyob yorqinlik". Vaqt. Olingan 3-noyabr, 2008.
- ^ "Jvaneng". Debsvana. Arxivlandi asl nusxasi 2012 yil 17 martda. Olingan 9 mart, 2012.
- ^ a b v Tixotskiy, J. (2000). Rossiyaning olmos koloniyasi: Saxa respublikasi. Yo'nalish. p. 254. ISBN 978-90-5702-420-7.
- ^ "Yahudiylar qimmatbaho toshlar savdosini hindularga topshirishadi". Spiegel Online. 2006 yil 15-may.
- ^ "Antverpen olmos markazining tarixi". Antverpen Jahon Diamond markazi. 2012-08-16.
- ^ "2001 yil 25 iyuldagi kontsentratsiyani umumiy bozor va EEA bitimiga mos kelishini e'lon qilgan komissiya qarori". Case No COMP / M.2333 - De Beers / LVMH. EUR-Lex. 2003.
- ^ "Biznes: o'zgaruvchan jihatlar; olmoslar". Iqtisodchi. 382 (8517): 68. 2007.
- ^ "Olmos sanoatida aniqlik bormi? Ballarni yig'ish uchun ehtiyot bo'ling - IDEX ning eslatmasi". idexonline.com. Olingan 24 sentyabr, 2014.
- ^ "Yaltiroq Sparcle". Gem & Jewellery eksportini rivojlantirish bo'yicha kengash. Arxivlandi asl nusxasi 2009 yil 16 iyunda. Olingan 26 aprel, 2009.
- ^ Even-Zohar, C. (2008 yil 6-noyabr). "De Beersdagi inqirozni yumshatish". DIB onlayn. Arxivlandi asl nusxasi 2011 yil 12 mayda. Olingan 26 aprel, 2009.
- ^ Hatto-Zohar, C. (1999 yil 3-noyabr). "De pivo olmos zaxirasini kamaytirish uchun". Milliy zargar. Arxivlandi asl nusxasi 2009 yil 5-iyulda. Olingan 26 aprel, 2009.
- ^ "Birinchi instansiya sudining 2007 yil 11 iyuldagi qarori - Alrosa v komissiyasi". EUR-Lex. 2007 yil. Olingan 26 aprel, 2009.
- ^ "Tog'-kon ishlari". De Beers guruhi. 2007. Arxivlangan asl nusxasi 2008 yil 13-iyunda. Olingan 4-yanvar, 2011.
- ^ "Olmos ishlab chiqaruvchisi Alrosa may oyida olmos sotish bozorini tiklaydi". RIA Novosti. 2009 yil 6-may. Olingan 25 may, 2009.
- ^ "Media-relizlar - Media Center - Alrosa". Alrosa. 2009 yil 22-dekabr. Arxivlangan asl nusxasi 2013 yil 20 avgustda. Olingan 4-yanvar, 2011.
- ^ "BHP uchun yana bir rekord foyda". ABC News. 2007 yil 22-avgust. Olingan 23 avgust, 2007.
- ^ "Bizning kompaniyalarimiz". Rio Tinto veb-sayti. Rio Tinto. Arxivlandi asl nusxasi 2013 yil 11-may kuni. Olingan 5 mart, 2009.
- ^ a b v Broadman, H. G.; Isik, G. (2007). Afrikaning ipak yo'li. Jahon banki nashrlari. 297-299 betlar. ISBN 978-0-8213-6835-0.
- ^ "Birjalar ro'yxati". Jahon brilliantlar federatsiyasi. Olingan 12 fevral, 2012.
- ^ "Shimoliy Amerika olmoslari savdosida pasayish belgisi yo'q". A & W olmoslari. Arxivlandi asl nusxasi 2009 yil 6-yanvarda. Olingan 5 may, 2009.
- ^ a b Pierson, Xyu O. (1993). Uglerod, grafit, olmos va fullerenlarga oid qo'llanma: xususiyatlari, qayta ishlanishi va qo'llanilishi. Uilyam Endryu. p. 280. ISBN 978-0-8155-1339-1.
- ^ a b Jeyms, Dunkan S. (1998). Antiqiy zargarlik buyumlari: uni ishlab chiqarish, materiallar va dizayn. Osprey nashriyoti. 82-102 betlar. ISBN 978-0-7478-0385-0.
- ^ "Olmoslarning klassik va maxsus shakllari". kristallsmolensk.com. Olingan 14 iyul, 2015.
- ^ Prelas, Mark Antonio; Popovici, Galina; Bigelou, Lui K. (1998). Sanoat olmoslari va olmosli plyonkalari bo'yicha qo'llanma. CRC Press. 984–992 betlar. ISBN 978-0-8247-9994-6.
- ^ "Marvaridni kesish". Mashhur mexanika. 74 (5): 760–764. 1940. ISSN 0032-4558.
- ^ Rapaport, Martin. "Olmos orzusini tirik tuting". Rapaport jurnali. Diamonds.net. Olingan 9 sentyabr, 2012.
- ^ a b JCK xodimlari (2011 yil 26-yanvar). "Sanoatni silkitadigan 10 narsa". JCK. Jckonline.com. Arxivlandi asl nusxasi 2013 yil 7-yanvarda. Olingan 9 sentyabr, 2012.
- ^ a b Epshteyn, EJ (1982). "Siz hech qachon olmos sotishga harakat qilganmisiz?". Atlantika. Olingan 5 may, 2009.
- ^ Bates, Rob (2011 yil 14-yanvar). "Forevermark bosh direktori bilan intervyu". JCK. Jckonline.com. Arxivlandi asl nusxasi 2012 yil 28 noyabrda. Olingan 9 sentyabr, 2012.
- ^ Xarlow, Jorj E. (1998). Olmosning tabiati. Kembrij universiteti matbuoti. p. 34. ISBN 978-0-521-62935-5.
- ^ Kogel, Jessica Elzea (2006). Sanoat minerallari va jinslari. Konchilik, metallurgiya va qidiruv ishlari bo'yicha jamiyat (AQSh). p. 416. ISBN 978-0-87335-233-8.
- ^ "Avstraliya olmos sanoati". Arxivlandi asl nusxasi 2009 yil 16-iyulda. Olingan 4 avgust, 2009.
- ^ Erlich, Edvard; Dan Xausel, V. (2002). Olmos konlari: kelib chiqishi, qidiruvi va kashfiyot tarixi. KO'K. p. 158. ISBN 978-0-87335-213-0.
- ^ "Olmos: Olmos minerallari haqidagi ma'lumotlar va rasmlar". minerallar.net. Olingan 24 sentyabr, 2014.
- ^ a b v "Olmoslarning sanoat statistikasi va ma'lumotlari". Amerika Qo'shma Shtatlarining Geologik xizmati. Olingan 5 may, 2009.
- ^ a b Nayza, K.E; Dismukes, JP (1994). Sintetik olmos: rivojlanayotgan CVD fan va texnologiyasi. Vili –IEEE. p. 628. ISBN 978-0-471-53589-8.
- ^ Xoltsapffel, C. (1856). Burilish va mexanik manipulyatsiya. Holtzapffel & Co. pp.https://archive.org/details/turningandmecha01holtgoog/page/n192 176]–178. ISBN 978-1-879335-39-4.
- ^ Koelo, R. T .; Yamada, S .; Aspinval, D. K .; Dono, M. L. H. (1995). "Alyuminiy asosli qotishmalarni burg'ulash va qayta ishlashda polikristalli olmos (PCD) asbob materiallarini MMC, shu jumladan burg'ulashda qo'llash". Mashinasozlik va ishlab chiqarish bo'yicha xalqaro jurnal. 35 (5): 761–774. doi:10.1016/0890-6955(95)93044-7.
- ^ Sakamoto, M .; Endriz, J.G .; Scifres, D.R. (1992). "Olmosli sovutgichga o'rnatilgan monolitik AlGaAs (800 nm) lazerli diodli massivdan 120 Vt quvvatli CW chiqish quvvati". Elektron xatlar. 28 (2): 197–199. doi:10.1049 / el: 19920123.
- ^ a b Yarnell, A. (2004). "Sun'iy olmoslarning ko'p qirralari". Kimyoviy va muhandislik yangiliklari. 82 (5): 26–31. doi:10.1021 / cen-v082n005.p026.
- ^ a b "Qarama-qarshi olmoslar". Birlashgan Millatlar. 21 mart 2001 yil. Arxivlangan asl nusxasi 2010 yil 9 martda. Olingan 5 may, 2009.
- ^ Catelle, W. R. (1911). Olmos. John Lane Co. p. 159.
- ^ a b v Xersi, V. (1940). Olmoslar kitobi. Nyu-York: Hearthside Press. 22-28 betlar. ISBN 978-1-4179-7715-4.
- ^ Ball, V. (1881). "1". Hindistonning olmoslari, oltinlari va ko'mirlari. London: Trübner & Co. p.1. Ball Britaniya xizmatida geolog edi.
- ^ "Pannadan eng katta olmos topildi". Bugun pochta. 2010 yil 1-iyul. Arxivlangan asl nusxasi 2011 yil 7-iyulda.
- ^ Shillington, K. (2005). Afrika tarixi ensiklopediyasi. CRC Press. p. 767. ISBN 978-1-57958-453-5.
- ^ a b Janse, A. J. A. (2007). "1870 yildan buyon global qo'pol olmos ishlab chiqarish". Toshlar va gemologiya. 43 (2): 98–119. doi:10.5741 / GEMS.43.2.98.
- ^ a b Lorenz, V. (2007). "G'arbiy Avstraliyadagi Argil: dunyodagi eng boy diamantifer quvur; uning o'tmishi va kelajagi". Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft. 56 (1–2): 35–40.
- ^ a b Kuk, Sara (2004 yil 17 oktyabr). "Montanada mikroskopik olmos topildi". Montana standarti. Arxivlandi asl nusxasi 2005 yil 21 yanvarda. Olingan 5 may, 2009.
- ^ Marshall, S .; Shore, J. (2004). "Olmos hayoti". Partizanlarning yangiliklar tarmog'i. Arxivlandi asl nusxasi 2007 yil 26 yanvarda. Olingan 21 mart, 2007.
- ^ Shigli, Jeyms E .; Chapman, Jon; Ellison, Robin K. (2001). "Argil olmos konining ochilishi va qazib olinishi, Avstraliya" (PDF). Toshlar va gemologiya. 37 (1): 26–41. doi:10.5741 / GEMS.37.1.26. Arxivlandi asl nusxasi (PDF) 2009 yil 30 sentyabrda. Olingan 20 fevral, 2010.
- ^ a b Basedau, M .; Mehler, A. (2005). Afrikaning Sahroi osti qismida siyosat. GIGA-Gamburg. 305-313 betlar. ISBN 978-3-928049-91-7.
- ^ Jahon olmos burjlari federatsiyasi (WFDB) va Olmos ishlab chiqaruvchilar xalqaro assotsiatsiyasi: 2000 yil 19 iyuldagi qo'shma qaror. Jahon olmos kengashi. 2000 yil 19-iyul. ISBN 978-90-04-13656-4. Olingan 5-noyabr, 2006.
- ^ "Kanadalik olmosli da'volarni tasdiqlash uchun ixtiyoriy odob-axloq qoidalari" (PDF). Kanadaning Diamond Code qo'mitasi. 2006 yil. Olingan 30 oktyabr, 2007.
- ^ Kjarsgaard, B. A .; Levinson, A. A. (2002). "Kanadadagi olmoslar". Toshlar va gemologiya. 38 (3): 208–238. doi:10.5741 / GEMS.38.3.208.
- ^ a b v "Global Diamond Industry: sir pardasini ko'tarish" (PDF). Bain & Company. Olingan 14 yanvar, 2012.
- ^ 1Shigley, J.E .; Abbosiy, Rizo; Shigli, Jeyms E. (2002). "Gemesis laboratoriyasi tomonidan yaratilgan olmoslar". Toshlar va gemologiya. 38 (4): 301–309. doi:10.5741 / GEMS.38.4.301.
- ^ Shigli, J.E .; Shen, Endi Xsi-Tien; Naslchilik, Kristofer M.; Makklur, Sheyn F.; Shigli, Jeyms E. (2004). "Laboratoriya Chatham tomonidan ishlab chiqarilgan rangli brilliant toshlarni yaratdi". Toshlar va gemologiya. 40 (2): 128–145. doi:10.5741 / GEMS.40.2.128.
- ^ Verner, M.; Locher, R (1998). "Doplanmagan va doping qilingan olmosli plyonkalarning o'sishi va qo'llanilishi". Fizikada taraqqiyot haqida hisobotlar. 61 (12): 1665–1710. Bibcode:1998RPPh ... 61.1665W. doi:10.1088/0034-4885/61/12/002.
- ^ Pisani, Bob (2012 yil 27-avgust). "Olmos biznesi, tog'-kon sanoati va chakana savdo". CNBC.
- ^ Kogel, J. E. (2006). Sanoat minerallari va toshlari. KO'K. 426-430 betlar. ISBN 978-0-87335-233-8.
- ^ O'Donoghue, M.; Joyner, L. (2003). Qimmatbaho toshlarni aniqlash. Buyuk Britaniya: Butterworth-Heinemann. 12-19 betlar. ISBN 978-0-7506-5512-5.
- ^ Barnard, A. S. (2000). Olmos formulasi. Butterworth-Heinemann. p. 115. ISBN 978-0-7506-4244-6.
- ^ Shigli, JE (2007). "Yangi qoplamali qimmatbaho toshlar bo'yicha kuzatishlar". Gemmologie: Zeitschrift der Deutschen Gemmologischen Gesellschaft. 56 (1–2): 53–56.
- ^ AQSh 4488821, Wenckus, J. F., "Simulyatsiya qilingan olmosni tabiiy olmosdan tez ajratish usuli va vositalari", 1984 yil 18-dekabrda nashr etilgan Ceres Electronics Corporation; AQSh Patenti 4.488.821
- ^ a b Edvards, H. G. M.; Chalmers, G. M (2005). Arxeologiya va san'at tarixidagi raman spektroskopiyasi. Qirollik kimyo jamiyati. 387-394 betlar. ISBN 978-0-85404-522-8.
- ^ a b Welbourn, C. (2006). "Sintetik olmoslarni aniqlash: hozirgi holat va kelajakdagi rivojlanish, 4-xalqaro gemologik simpozium materiallari". Toshlar va gemologiya. 42 (3): 34–35.
- ^ Donahue, PJ (2004 yil 19 aprel). "DTC DiamondSure va DiamondView-ning GIA distribyutorini tayinladi". Professional zargarlar jurnali. Olingan 2 mart, 2009.
- ^ "SSEF olmosli spotter va SSEF yoritgichi". SSEF Shveytsariya gemmologik instituti. Arxivlandi asl nusxasi 2009 yil 27 iyunda. Olingan 5 may, 2009.
- ^ "Belgiya aeroportidagi olmosli g'ilofdan 50 million dollardan ortiq qamoqqa olingan". BBC yangiliklari. 2013 yil 8-may.
- ^ "Kim, nima, nima uchun: o'g'irlangan olmosni qanday aniqlash mumkin?". BBC yangiliklari. 2013 yil 21-fevral.
- ^ "Bryusseldagi olmosni talash to'rlari" ulkan "yo'l". BBC yangiliklari. 2013 yil 19-fevral.
- ^ Liddell, XG; Skott, R. "Adamas". Yunoncha-inglizcha leksika. Perseus loyihasi.
- ^ Katta Pliniy (2004). Tabiiy tarix: tanlov. Pingvin kitoblari. p. 371. ISBN 978-0-14-044413-1.
- ^ "Xitoy birinchi marta olmosdan foydalangan". BBC yangiliklari. 2005 yil 17-may. Olingan 21 mart, 2007.
- ^ Qarang:
- Lavuazye (1772) "Premer mémoire sur la destruktsiya du diamant par le feu" (Olmosni olov bilan yo'q qilish to'g'risida birinchi esdalik), Histoire de l'Académie royale des fanlar. Avec les Mémoires de Mathématique & de Physique (Qirollik Fanlar akademiyasi tarixi. Matematika va fizika xotiralari bilan), 2-qism, 564-591.
- Lavuazye (1772) "Second mémoire sur la destroy du diamant par le feu" (Olmosni olov bilan yo'q qilish to'g'risida ikkinchi xotira), Histoire de l'Académie royale des fanlar. Avec les Mémoires de Mathématique & de Physique, 2-qism, 591-616.
- ^ Smitson Tennant (1797) "Olmosning tabiati to'g'risida" London Qirollik Jamiyatining falsafiy operatsiyalari, 87 : 123–127.
Kitoblar
- C. Hatto-Zohar (2007). Menikidan xo'jayinga: xalqaro olmos sanoatida korporativ strategiyalar va hukumat siyosati (2-nashr). Mining Journal Press.
- G. Devies (1994). Olmosning xususiyatlari va o'sishi. INSPEC. ISBN 978-0-85296-875-8.
- M. O'Donoghue (2006). Toshlar. Elsevier. ISBN 978-0-7506-5856-0.
- M. O'Donoghue va L. Joyner (2003). Qimmatbaho toshlarni aniqlash. Buyuk Britaniya: Butterworth-Heinemann. ISBN 978-0-7506-5512-5.
- A. Feldman va L.X. Robins (1991). Olmos plyonkalari va tegishli materiallarning qo'llanilishi. Elsevier.
- J.E. Field (1979). Olmosning xususiyatlari. London: Academic Press. ISBN 978-0-12-255350-9.
- J.E. Field (1992). Tabiiy va sintetik olmosning xususiyatlari. London: Academic Press. ISBN 978-0-12-255352-3.
- V. Xersi (1940). Olmoslar kitobi. Hearthside Press Nyu-York. ISBN 978-1-4179-7715-4.
- S. Koizumi, CE Nebel va M. Nesladek (2008). Olmos kasalligi fizikasi va qo'llanishi. Vili VCH. ISBN 978-3-527-40801-6.
- L.S. Pan va D.R. Kani (1995). Olmos: elektron xususiyatlar va ilovalar. Kluwer Academic Publishers. ISBN 978-0-7923-9524-9.
- Pagel-Tizen, Verena (2001). Diamond Grading ABC: qo'llanma. Antverpen: Rubin va O'g'il. ISBN 978-3-9800434-6-5.
- R.L.Radovich, P.M. Walker va P.A. Tashlovchi (1965). Uglerod kimyosi va fizikasi: qator yutuqlar. Nyu-York: Marsel Dekker. ISBN 978-0-8247-0987-7.
- M. Tolkovskiy (1919). Olmos dizayni: Olmosdagi yorug'likning aksi va sinishini o'rganish. London: E. & F.N. Spon.
- RW Wise (2016). Qimmatbaho toshlar savdosi sirlari: Qimmatbaho toshlar uchun biluvchilar uchun qo'llanma (Ikkinchi nashr). Brunsvik uyi matbuoti. ISBN 9780972822329.
- A.M. Zaytsev (2001). Olmosning optik xususiyatlari: ma'lumotlar bo'yicha qo'llanma. Springer. ISBN 978-3-540-66582-3.
Tashqi havolalar
- Olmosning xususiyatlari: Ioffe ma'lumotlar bazasi
- "Olmos paydo bo'lishida ko'k floresanni tushunishga hissa qo'shish". (2007) Amerika Gemologik Instituti (GIA)
- Tayson, Piter (2000 yil noyabr). "Osmondagi olmoslar". Qabul qilingan 2005 yil 10 mart.
- Olmos sotishga harakat qilganmisiz?


