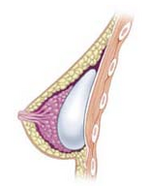
Ko'krak implantatsiyasi - Breast implant
| Ko'krak implantatsiyasi | |
|---|---|
Implantatlar uchun ko'krak qafasini belgilab qo'ygan shifokor videosi. | |
| Mutaxassisligi | Plastik jarrohlik |
A ko'krak implantatsiyasi a protez odamning o'lchamini, shakli va konturini o'zgartirish uchun ishlatiladi ko'krak. Rekonstruktiv plastik jarrohlik, a dan keyin tabiiy ko'rinadigan ko'krakni tiklash uchun ko'krak implantlarini joylashtirish mumkin mastektomiya yoki tuzatish uchun tug'ma nuqsonlar va deformatsiyalar ko'krak devorining. Ular shuningdek, ko'krak ko'rinishini kattalashtirish uchun kosmetikadan foydalaniladi ko'krakni kattalashtirish bo'yicha operatsiya.
Implantlarning asoratlari o'z ichiga olishi mumkin ko'krak og'rig'i, terining o'zgarishi, infektsiya, yorilish va ko'krak atrofida suyuqlik to'planishi.[1]
To'ldiruvchi moddasi bilan aniqlangan to'rtta ko'krak implantlarining umumiy turlari mavjud: fiziologik eritma, silikon jel, tuzilgan va kompozitsion plomba moddasi. Tuzli implant an elastomer silikon steril bilan to'ldirilgan qobiq fiziologik eritma jarrohlik paytida; silikon implantatida viskoz bilan oldindan to'ldirilgan elastomer silikon qobig'i mavjud silikon jel; tuzilgan implantlarda ichki elastomer silikon chig'anoqlari va ikkita sho'r suv bilan to'ldirilgan lümen ishlatiladi; va muqobil kompozitsion implantlar kabi turli xil plomba moddalari, masalan soya yog'i yoki polipropilen ip. Kompozit implantatlar odatda endi foydalanishga tavsiya etilmaydi va aslida sog'liq uchun xavfli va asoratlari tufayli Qo'shma Shtatlar va Evropada ulardan foydalanish taqiqlangan.
Jarrohlik amaliyotida ko'krakni tiklash uchun to'qima kengaytiruvchisi qurilma kelajakda doimiy ko'krak implantatsiyasi uchun implant cho'ntagini shakllantirish va o'rnatish uchun ishlatiladigan vaqtinchalik ko'krak protezidir. Erkaklarning ko'krak qusurlari va deformatsiyalarini tuzatish uchun pektoral implant - bu erkakning ko'krak devorini tiklash va estetik tiklash uchun ishlatiladigan ko'krak protezidir (qarang: jinekomastiya va mastopeksiya ).
Foydalanadi
A mammoplastika ko'krak implantatsiyasini amalga oshirish vositalarini joylashtirish tartibi uchta (3) maqsadga ega:
- birlamchi rekonstruksiya: travma natijasida zararlangan ko'krak to'qimalarini almashtirish (to'mtoq, kirib boruvchi, portlash ), kasallik (ko'krak bezi saratoni ) va muvaffaqiyatsiz anatomik rivojlanish (tuberöz ko'krak deformatsiyasi ).
- qayta ko'rib chiqish va rekonstruksiya qilish: ko'krakni qayta tiklash bo'yicha oldingi operatsiya natijalarini qayta ko'rib chiqish (to'g'rilash).
- birlamchi kattalashtirish: estetik jihatdan kattalashtirish ko'krak hajmi, shakli va hissi.
The operatsiya xonasi (Yoki) post vaqti -mastektomiya ko'krakni qayta tiklash va of ko'krakni kattalashtirish jarrohlik qo'llaniladigan protsedura, kesmalar turi, ko'krak implantatsiyasi (turi va materiallari) va implant cho'ntagining ko'krak mintaqasi bilan belgilanadi.
Yaqinda o'tkazilgan tadqiqotlar shuni ko'rsatdiki, ko'krak qafasi jarrohligi, shu jumladan ko'krak implantatsiyasi, kattalashtirish, mastopeksiya va ko'krak qisqarishi bilan og'rigan bemorlarda mammogrammalar odatdagi protsedurada qo'llanilgandan yuqori chastotada o'tkazilmasligi kerak.[2]
Psixologiya
The ko'krakni kattalashtirish bemor odatda shaxsiy ayolning tashqi qiyofasi va tanasi bilan bog'liq psixologik bezovtalikni ko'rsatadigan yosh ayoldir o'z qiyofasi va bu haqda tanqidlarga (masxara qilishga) chidaganlik tarixi estetika uning shaxsidan.[3] Tadqiqotlar Tana qiyofasi Ko'krakni ko'paytiradigan bemorlar (2003) va Tana dismorfik buzilishi va kosmetik jarrohlik (2006) operatsiya qilingan ayol haqida xabar bergan ko'krakni kattalashtirish jarrohlik ham o'tkazildi psixoterapiya, past darajada azob chekdi o'z-o'zini hurmat, tez-tez sodir bo'lishini taqdim etdi psixologik tushkunlik, urinib ko'rgan o'z joniga qasd qilish va azob chekdi tana dismorfiyasi, ruhiy kasallikning bir turi.
Operatsiyadan keyingi bemorlarda ruhiy salomatlik va hayot sifati bo'yicha o'tkazilgan so'rovlar, yaxshilangan jismoniy sog'liq, tashqi ko'rinish, ijtimoiy hayot, o'ziga bo'lgan ishonch, o'ziga hurmat va qoniqarli jinsiy faoliyat. Bundan tashqari, ayollar ko'krak implantatsiyasi natijalaridan uzoq muddatli qoniqish haqida xabar berishdi; ba'zilari tibbiy asoratlarni boshdan kechirganiga qaramay, jarrohlik yo'li bilan qayta ko'rib chiqishni talab qiladi, yoki tuzatish yoki estetik. Xuddi shunday, Daniyada ham 8 foiz ko'krakni kattalashtirish bemorlar operatsiyadan oldin psixiatriya kasalxonasiga yotqizilganlar.[4][5][6][7][8][9][10][11][12]
2008 yilda, uzunlamasına o'rganish Ko'krak bezi implantatsiyasiga ega ayollar orasida o'z joniga qasd qilish va o'limning boshqa tashqi sabablaridan ortiqcha o'lim (2007), ko'krak bezi implantlarini izlagan ayollar, bu sodir etish ehtimoli deyarli 3 baravar yuqori ekanligini xabar qilishdi o'z joniga qasd qilish singari ko'krak implantlarini izlamagan ayollar singari. Oddiy o'z joniga qasd qilish darajasi bilan taqqoslaganda, ko'kragi ko'paygan ayollar uchun o'z joniga qasd qilish darajasi implantatsiyadan keyingi 10 yilgacha doimiy bo'lib qoldi, ammo bu 11 yillik davrda 4,5 baravarga oshdi va shuning uchun implantatsiyadan keyingi 20 yillik davrda 6 baravar ko'paygan 19 yilgacha saqlanib qoldi. Bundan tashqari, o'z joniga qasd qilish xavfi bilan bir qatorda, ko'krak implantlari bo'lgan ayollar ham o'lim xavfi bilan duch kelishgan alkogolizm retsept bo'yicha va rekreatsion dorilarni suiiste'mol qilish.[13][14] Ettita tadqiqot statistik ravishda ayolning ko'kragini ko'paytirishni o'z joniga qasd qilish darajasi bilan bog'lab qo'ygan bo'lsa-da, tadqiqotlar shuni ko'rsatadiki, ko'krakni ko'paytirish bo'yicha operatsiya o'lim darajasini oshirmaydi; va birinchi navbatda, bu psixopatologik duchor bo'lish ehtimoli yuqori bo'lgan ayol ko'krakni kattalashtirish protsedura.[15][16][17][18][19][20]
O'qish Ko'krakni kattalashtirish mammoplastikasining o'z qadr-qimmati va jinsiy hayotga ta'siri: miqdoriy tahlil (2007), ayollar o'zlarining yaxshilanganligini ta'kidladilar o'z qiyofasi, o'z-o'zini hurmat va ko'krakni kattalashtirish uchun qoniqarli jinsiy faoliyatning ko'payishi; 21-57 yoshdagi kogorta, operatsiyadan keyingi o'zini o'zi qadrlashning o'rtacha o'sishi, 30 ball bo'yicha 20,7 dan 24,9 ballgacha o'zgargan Rozenbergning o'zini o'zi qadrlash shkalasi, bu ma'lumotlar ayolning 78,6 foizga o'sishini qo'llab-quvvatladi libido, uning operatsiyadan oldingi libido darajasiga nisbatan.[21] Shuning uchun, har qanday operatsiyaga rozi bo'lishdan oldin, plastik jarroh ayolni baholaydi va ko'rib chiqadi ruhiy salomatlik ko'krak implantlari uning qadr-qimmatiga ijobiy ta'sir ko'rsatishi yoki yo'qligini aniqlash jinsiy faoliyat.
Asoratlar
The plastik jarrohlik ko'krak implantatsiyalash moslamalarini joylashtirish yoki ko'krakni qayta tiklash yoki uchun estetik maqsad, umumiy sog'liq uchun bir xil xavflarni keltirib chiqaradi jarrohlik kabi salbiy reaktsiya kabi behushlik, gematoma (operatsiyadan keyingi qon ketish), kech gematoma (operatsiyadan keyingi qonash 6 oy va undan ko'p vaqt o'tgach),[22] seroma (suyuqlik to'planishi), kesma joyining parchalanishi (yara infektsiyasi). Ko'krakni kattalashtirishga xos bo'lgan asoratlar orasida ko'krak og'rig'i, hissiyotning o'zgarishi, emizishdagi to'siq, ko'zga ko'rinadigan ajinlar, assimetriya, ko'krak to'qimalarining ingichkalashi va simmastiya, ko'krak orasidagi tabiiy tekislikni to'xtatadigan büstning "noni". Uyda joylashtirilgan implantlarni asoratlarini davolashning o'ziga xos usullari -kapsula kontrakturasi va kapsulalarning yorilishi - davriydir MRI monitoring va fizik tekshiruvlar. Bundan tashqari, asoratlar va implantatsiya operatsiyasi bilan bog'liq bo'lgan qayta operatsiyalar va to'qima kengaytirgichlari (operatsiya paytida joylashtiradigan implantlar) noxush holatga olib kelishi mumkin yara izlari bemorlarning taxminan 6-7 foizida.[23][24][25] Statistik jihatdan, Kosmetik implantatsiya qilingan ayollarning 20 foizi va ko'krakni qayta tiklash implantatsiyasini o'tkazgan ayollarning 50 foizi 10 yil ichida eksplantatsiyani talab qildilar.[26]
Xavfsizlik
1990-yillarning boshlarida, ro'yxatdagi mamlakatlarning milliy sog'liqni saqlash vazirliklari tomonidan ko'krak silikon-gel implantlari va tizimli va avto-immunitet kasalliklari o'rtasidagi sababiy bog'liqlik bo'yicha tegishli tadqiqotlar ko'rib chiqildi. Kollektiv xulosa shundan iboratki, ko'krak silikon implantlarini implantatsiyasi bilan kasallikning har ikkala turi o'rtasida sababiy bog'liqlikni o'rnatadigan dalillar mavjud emas. Daniya tadqiqotlari Daniyalik ayollarning ko'krak silikon implantlari bilan uzoq muddatli salomatlik holati (2004), o'rtacha 19 yil davomida ko'krak qafasini joylashtirgan ayollar, haddan tashqari ko'pligi haqida xabar berishlari mumkin emasligini xabar qilishdi revmatik kasallik alomatlar nazorat guruhidagi ayollarga qaraganda.[27] Keyingi tadqiqot Mammoplastika bilan og'rigan bemorlarning ko'payishi o'rtasidagi o'lim darajasi: yangilanish (2006) pasayganligi haqida xabar bergan o'limning standartlashtirilgan darajasi va xavfining ortishi o'pka saratoni boshqa turdagi plastik jarrohlik operatsiyalariga qaraganda, ko'krak implantatsiyalangan bemorlar orasida o'lim; o'lim darajasi farqlari bilan bog'liq edi tamaki chekish.[28] O'qish Kosmetik ko'krak implantatsiyasiga ega bo'lgan Kanada ayollari orasida o'lim (2006), taxminan 25000 ta ko'krak implantatsiyasiga ega ayollar, ular orasida ko'krak bezi saratonining umumiy populyatsiyaga nisbatan 43 foizga pastligi va saraton kasalligining o'rtacha darajadan pastligi haqida xabar berishdi.[29]
| Yil | Mamlakat | Tizimli ko'rib chiqish guruhi | Xulosa |
|---|---|---|---|
| 1991–93 | Birlashgan Qirollik | Mustaqil ekspertlar maslahat guruhi (IEAG) | Silikon-jel ko'krak implantatsiyasini o'tkazgan bemorlarda biriktiruvchi to'qima kasalligi xavfi oshganligi va Buyuk Britaniyada ko'krak implantatsiyasi amaliyoti yoki siyosatini o'zgartirish uchun hech qanday sabab yo'q edi. |
| 1996 | Qo'shma Shtatlar | AQSh Tibbiyot Instituti (XMT)[30] | "Belgilangan biriktiruvchi to'qima kasalligi bilan silikon jeli yoki fiziologik eritma bilan to'ldirilgan ko'krak implantlarini assotsiatsiyasi uchun dalillar etarli emas". |
| 1996 | Frantsiya | Agence Nationale pour le Developpement de l'Evaluation Medicale (ANDEM) [Tibbiyotni rivojlantirish va baholash milliy agentligi][31] | Frantsuzcha asl nusxa: "Nous n'avons pas observé de connectivité ni d'autre patologie auto-immun sezgir d'être directement ou indirectement induite par la présence d'un implant mammaire en particulier en gel de silikon ...." Ingliz tilidagi tarjimasi: "Biz biriktiruvchi to'qima kasalliklarini ko'krak implantatsiyasi, xususan, silikon jeldan biri bilan bevosita yoki bilvosita bog'liqligini kuzatmadik." |
| 1997 | Avstraliya | Terapevtik asboblarni baholash qo'mitasi (TDEC) | "Hozirgi va yuqori sifatli adabiyotlar shuni ko'rsatadiki, ko'krak implantlari va biriktiruvchi to'qima kasalligiga o'xshash sindromlar (atipik biriktiruvchi to'qima kasalliklari) o'rtasida bog'liqlik yo'q."[32] |
| 1998 | Germaniya | Federal tibbiyot va tibbiy mahsulotlar instituti | "Silikon ko'krak implantlari avtomatik immunitet kasalliklarini yoki revmatik kasalliklarni keltirib chiqarmaydi va homiladorlik, emizish qobiliyati va emizikli bolalarning sog'lig'iga salbiy ta'sir ko'rsatmaydi. Silikon allergiya borligi to'g'risida ilmiy dalillar yo'q , silikon zaharlanishi, atipik silikon kasalliklari yoki yangi silikon kasalligi. "[33] |
| 2000 | Qo'shma Shtatlar | Federal sud buyrug'i bilan ko'rib chiqish[34] | "Ayniqsa, silikon-gel bilan to'ldirilgan ko'krak implantlari va har qanday individual CTDlar, aniq birlashtirilgan CTDlar yoki boshqa avtomatik immunitetli yoki revmatik holatlar o'rtasida bog'liqlik yo'q." |
| 2000 | Yevropa Ittifoqi | Plastik jarrohlikda tibbiy ta'minot va sifatni ta'minlash bo'yicha Evropa qo'mitasi (EQUAM) | "Qo'shimcha tibbiy tadqiqotlar silikon-gel bilan to'ldirilgan ko'krak implantlari va an'anaviy avto-immunitetli yoki biriktiruvchi to'qima kasalliklari o'rtasida hech qanday bog'liqlik mavjud emas. saraton, va boshqa biron bir xavfli kasallik. . . . EQUAM silikon allergiyasi, silikon intoksikatsiyasi, atipik kasallik yoki "yangi silikon kasalligi" mavjudligiga oid ilmiy dalillar yo'qligiga ishonishda davom etmoqda. "[35] |
| 2001 | Birlashgan Qirollik | Buyuk Britaniyaning mustaqil sharh guruhi (UK-IRG) | "Anormal immun javob yoki odatiy yoki atipik biriktiruvchi to'qima kasalliklari yoki sindromlari bilan bog'liqlik haqida hech qanday ma'lumot yo'q."[36] |
| 2001 | Qo'shma Shtatlar | Sud tomonidan tayinlangan Milliy Ilmiy Kengashni ko'rib chiqish[37] | Panel, biriktirilgan to'qima kasalliklarini (KTD) baholab, ko'krak implantlari va ushbu KTDlar o'rtasida sababiy dalillar yo'qligini xulosa qildi. |
| 2003 | Ispaniya | Ilm-fan va texnologiya imkoniyatlarini baholash (STOA) | STOA-ning Evropa Parlamenti Petitsiya qo'mitasiga bergan hisobotida, hozirgi ilmiy dalillar SBI [ko'krak silikon implantlari] ni og'ir kasalliklarga, masalan: ko'krak bezi saratoni, biriktiruvchi to'qima kasalliklari.[38] |
| 2009 | Yevropa Ittifoqi | Xalqaro sifatni ta'minlash, tibbiy texnologiyalar va plastik jarrohlik asboblari paneli (IQUAM) | Transatlantic Innovations konferentsiyasining (2009 yil aprel) konsensus bayonotida ko'rsatilishicha, qo'shimcha tibbiy tadqiqotlar silikon jel bilan to'ldirilgan ko'krak implantlari va karsinomasi yoki metabolik, immunitet yoki allergik kasalliklar bilan bog'liqligi yo'q.[39] |
Implantatsiya yorilishi
Chunki ko'krak implantatsiyasi bu a III sinf tibbiy asbob cheklangan mahsulotning ishlash muddati, yorilish tezligining asosiy omillari uning yoshi va dizayni; Shunga qaramay, ko'krak implantatsiyalash moslamasi mexanik butunligini ayol tanasida o'nlab yillar davomida saqlab turishi mumkin.[40] Tuzli ko'krak implantatsiyasi yorilib, oqib chiqsa va bo'shab qolsa, u tezda deflatsiyalanadi va shu bilan oson tushuntirish mumkin (jarrohlik yo'li bilan olib tashlash). Keyingi hisobot, Natrelle fiziologik eritma bilan to'ldirilgan ko'krak qafasi: 10 yillik istiqbolli tadqiqot (2009) implantatsiyadan keyingi 3 yillik jarrohlik-deflyatsiya stavkalarini 3-5 foizni va implantatsiyadan keyingi 10 yillik jarrohlik-deflyatsiya stavkalarini 10 yildan keyin ko'rsatdi.[41]

Silikon ko'krak implantatsiyasi yorilib ketganda, u odatda defiltatsiyalanmaydi, ammo plomba jeli undan implantatsiya cho'ntagiga o'tishi mumkin; shuning uchun intrakapsular yorilish (kapsuladagi oqish) ekstrakapsular yorilishga aylanishi mumkin (kapsuladan tashqarida oqish) va har bir hodisa eksplantatsiya yo'li bilan hal qilinadi. Sızdıran silikon plomba-gel ko'krak to'qimalaridan ayol tanasining boshqa joylariga ko'chib o'tishi mumkin bo'lsa-da, ko'pincha klinik asoratlar bilan cheklangan ko'krak va qo'ltiq maydonlar, odatda sifatida namoyon bo'ladi granulomalar (yallig'lanish tugunlari) va aksillar limfadenopatiya (kattalashtirilgan limfa bezlari qo'ltiq sohasida).[42][43][44]
Ko'krak implantatsiyasi yorilishining shubhali mexanizmlari quyidagilardir:
- implantatsiya paytida zarar
- (boshqa) jarrohlik muolajalar paytida shikastlanish
- ko'krak implantatsiyasi qobig'ining kimyoviy degradatsiyasi
- travma (ochiq jarohat, penetratsion travma, portlash travması )
- an'anaviy mexanik bosim mamografik ko'krak bezi tekshiruvi [45]
Silikon implantatsiyasining yorilishini magnit-rezonans tomografiya yordamida baholash mumkin; uzoq muddatli istiqboldan MRI bitta lümenli ko'krak implantlari uchun ma'lumotlar, ikkinchi avlod silikon-jel ko'krak implantlari haqidagi Evropa adabiyotlarida (1970-yillarning dizayni), implantatsiyadan keyingi 10 yillik davrda (15-30%) jimjitlik bilan yorilish tezligi 8-15 foizni tashkil etganligi haqida xabar berilgan. bemorlar).[46][47][48][49]
O'qish Mentorning MemoryGel implantlarining xavfsizligi va samaradorligi 6 yoshda (2009), bu AQSh FDA ning yadrosini o'rganish edi klinik sinovlar birlamchi uchun ko'krakni kattalashtirish jarrohlik operatsiyalari bilan shug'ullanadigan bemorlar, implantatsiyadan keyingi 6 yillik operatsiyani o'tkazishda asbobning yorilishi darajasi 1,1 foizni tashkil etganligini xabar qilishdi.[50] Ning birinchi seriyasi MRI qalin plomba-gel bilan silikon ko'krak implantlarini baholashda o'rtacha 6 yoshga to'lgan qurilmada 1 foiz yoki undan kam bo'lgan yorilish tezligi qayd etildi.[51] Statistik ma'lumotlarga ko'ra, ayolning qo'lda tekshiruvi (palpatsiya) ko'krak bezi yirtilib ketganligini aniq baholash uchun etarli emas. O'qish, Silikon ko'krak implantatsiyasining yorilishi diagnostikasi: Klinik natijalar magnit-rezonans tomografiya natijalari bilan taqqoslaganda (2005), asemptomatik bemorlarda, yorilgan ko'krak implantlarining atigi 30 foizini tajribali plastik jarroh aniq paypaslaydi va aniqlaydi, MRT tekshiruvlari ko'krak implantlari yorilishlarining 86 foizini aniq aniqlaydi.[52] Shu sababli, AQSh FDA tomonidan implantatsiyadan keyingi 3 yillik belgidan boshlab, so'ngra har ikki yilda bir marta, rejali MRI tekshiruvlari tavsiya qilindi.[23] Shunga qaramay, AQShdan tashqarida, boshqa millatlarning tibbiyot muassasalari muntazam ravishda MRI skrining tekshiruvini ma'qullamadilar va uning o'rniga bunday tekshiruvni taklif qildilar radiologik tekshiruv ikki maqsad uchun ajratilishi kerak: (i) ko'krak implantatsiyasining uzilishiga shubha qilingan ayol uchun; va (ii) tasdiqlash uchun mamografik va ultratovushli yorilgan ko'krak implantatsiyasi mavjudligini ko'rsatadigan tadqiqotlar.[53]
Bundan tashqari, Silikon ko'krak implantatsiyasining yorilishini aniqlash uchun magnit-rezonans tomografiya diagnostikasining aniqligiga tadqiqot natijalarini ta'siri: meta-tahlil (2011) asemptomatik ayollarning ko'krak skriningi MRGlari ko'krak implantatsiyasining yorilishi holatlarini yuqori baholashi mumkinligi haqida xabar bergan.[54] Tadbirda AQSh oziq-ovqat va farmatsevtika idorasi «ko'krak implantlari hayot davomida ishlatiladigan vositalar emasligini ta'kidladi. Ayolning ko'krak qafasi silikonli gel bilan to'ldirilganligi qancha ko'p bo'lsa, u asoratlarni boshdan kechiradi ».[55]
Kapsular kontraktura
Inson tanasi immunitet reaktsiyasi jarrohlik yo'li bilan o'rnatilgan begona narsaga - ko'krak implantatsiyasiga, yurakka yurak stimulyatori, ortopedik protez - uni kapsulalash kerak chandiq to'qimasi mahkam to'qilgan kapsulalar kollagen begona narsalarni izolyatsiya qilish orqali tananing yaxlitligini saqlab qolish va shuning uchun uning mavjudligiga toqat qilish uchun tolalar. Kapsular kontraktura - bu normal kapsula to'qimasidan ajralib turishi kerak - kollagen-tolali kapsula qalinlashganda va ko'krak implantatsiyasini siqib chiqarganda paydo bo'ladi; bu og'riqli asorat bu ko'krak implantatsiyasini yoki ko'krakni yoki ikkalasini ham buzishi mumkin.

Kapsül kontrakturasının sababi noma'lum, ammo kasallikning keng tarqalgan omillari orasida bakterial ifloslanish, qurilma qobig'ining yorilishi, plomba oqishi va gematoma. Kapsül kontrakturasini kamaytirishni kamaytiradigan jarrohlik implantatsiya protseduralariga mushak osti bo'shliqlari, yuzasi teksturali (poliuretan bilan qoplangan) ko'krak implantlarini qo'llash kiradi;[56][57][58] implantlar bilan operatsiyadan oldin cheklangan ishlov berish, ko'krak implantatsiyasini joylashtirishdan oldin implantatsiya cho'ntagining ko'krak terisi bilan cheklangan aloqa va qabul qiluvchi joyni uch karra antibiotikli eritmalar bilan sug'orish.[59][60]
Kapsulali kontrakturani tuzatish uchun kollagen-tolali kapsulani ochiq kapsulomiyasi (jarrohlik yo'li bilan) yoki ko'krak implantatsiyasini olib tashlash va uni almashtirishni talab qilishi mumkin. Bundan tashqari, kapsula kontrakturasini davolashda, yopiq kapsulomiya (tashqi manipulyatsiya orqali buzilish) bir paytlar qattiq kapsulalarni davolash uchun odatiy manevr bo'lgan, ammo endi ko'ngilsiz usul, chunki u ko'krak implantatsiyasini yorishi mumkin. Kollagen-tolali kapsulalarni jarrohlik bo'lmagan davolash usullari massajni o'z ichiga oladi ultratovushli terapiya, leykotrien yo'lining inhibitörleri kabi zafirlukast (Accolate) yoki montelukast (Singulair) va impulsli elektromagnit maydon terapiyasi (PEMFT).[61][62][63][64]
Ta'mirlash va qayta ko'rib chiqish operatsiyalari
Bemor kattalashtirish mammoplastikasi natijalaridan qoniqmasa; yoki texnik yoki tibbiy asoratlar yuzaga kelganda; yoki ko'krak implantlarining mahsulot muddati cheklanganligi sababli, ehtimol u ko'krak implantlarini almashtirishni talab qilishi mumkin. Oddiy revizion jarrohlik ko'rsatmalariga katta va kichik tibbiy asoratlar, kapsula kontrakturasi, qobiqning yorilishi va qurilma deflyatsiyasi.[45] Mastektomiyadan so'ng yumshoq to'qimalarda va ko'krak terisining konvertida va ko'krak qafasidagi o'zgarishlar tufayli ko'krakni qayta tiklaydigan bemorlar uchun revizyon tezligi ko'proq edi. anatomik ko'krak chegaralari, ayniqsa tashqi yordamchi dori olgan ayollarda radiatsiya terapiyasi.[45] Bundan tashqari, ko'krakni tiklashdan tashqari, ko'krak bezi saratoni bemorlar odatda tabiiy ko'rinish, o'lcham, shakl va hissiyot büstü yaratish uchun ko'krak-areola kompleksini (NAC) qayta ko'rib chiqish operatsiyasini va qarama-qarshi ko'krak ustidagi simmetriya protseduralarini o'tkazadilar. Bemorning pektoral yumshoq to'qimalarining xususiyatlariga ko'krak implantlarining turi va o'lchamlarini diqqat bilan moslashtirish revizion jarrohlik holatlarini kamaytiradi. Tegishli to'qimalarga mos kelish, implantlarni tanlash va to'g'ri implantatsiya texnikasi, 7 yillik ko'rsatkich bo'yicha qayta operatsiya darajasi 3 foizni tashkil etdi, bu haqda 3 yil belgisida qayta ishlash darajasi 20 foizga nisbatan. AQSh oziq-ovqat va farmatsevtika idorasi.[65][66]
Tizimli kasallik

1990-yillarning boshidan beri bir qator mustaqil tizimli kompleks tekshiruvlar ko'krak silikon gel implantlari va tizimli kasalliklar da'volari o'rtasidagi bog'liqlik bo'yicha ishlarni o'rganib chiqdi. Ushbu sharhlarning yakdilligi (quyida Ko'krak bezi implantlarining xavfsizligi sarlavhasi ostida ko'rsatilgan) shundan iboratki, fiziologik eritma yoki silikon ko'krak implantatsiyalari bilan tizimli kasallik implantatsiyasi o'rtasida biron bir bog'liqlik mavjudligiga oid dalillar mavjud emas Ushbu muammoni o'rganib chiqqandan so'ng, AQSh FDA bilan kelishilgan va yana bir bor "adabiyotda chop etilgan epidemiologik dalillarning og'irligi fibromiyalgiya va ko'krak implantlari o'rtasidagi aloqani qo'llab-quvvatlamasligini" tasdiqladi. Lipvort tomonidan keng qamrovli tizimli sharh (2011) [67] "kosmetik ko'krak implantlari va CTDlar o'rtasidagi bog'liqlik bo'yicha qolgan har qanday da'volar ilmiy adabiyotlar tomonidan qo'llab-quvvatlanmaydi" degan xulosaga keladi.
Platin toksikligi
Platina tibbiyotda ishlatiladigan silikon implant polimer chig'anoqlari va boshqa silikon moslamalarni tayyorlashda ishlatiladigan katalizator hisoblanadi. Adabiyot shuni ko'rsatadiki, ushbu implantlardan platinaning oz miqdordagi oqishi (oqishi) va atrofdagi to'qimalarda mavjud. FDA 2002 yilda platina va ko'krak implantlari bo'yicha tibbiy adabiyotlardan olingan tadqiqotlarni ko'rib chiqdi va implantatsiya qilingan bemorlarda platinadan toksikligini ko'rsatadigan juda kam dalillar mavjud degan xulosaga keldi.[68] FDA bir necha yil o'tgach, ushbu tadqiqotni va qo'shimcha adabiyotlarni qayta ko'rib chiqdi va implantlarda ishlatiladigan platina katalizatorlari ionlashtirilmaganligi va shuning uchun ayollar uchun xavf tug'dirmasligi haqidagi oldingi xulosalarini tasdiqladi.[69]
Anaplastik yirik hujayrali limfoma
FDA ko'krak bezi implantatsiyasining saraton kasalligining kamdan-kam uchraydigan shakli bilan bog'liq bo'lishi mumkinligini aniqladi anaplastik katta hujayrali limfoma, surunkali bakterial yallig'lanish bilan bog'liq deb ishoniladi.[70] Shu kabi ALCL hodisalari tibbiy implantlarning boshqa turlari, shu jumladan qon tomirlari kirish portlari, ortopedik kestirib, implantlar va jag '(TMJ) implantlari bilan kuzatilgan. 2015 yilda plastik jarrohlar 79 ta bemorga bag'ishlangan adabiyotdagi 37 ta maqolani ko'rib chiqdilar va yana 94 ta ro'yxatdan o'tmagan holatlarni to'pladilar, natijada ko'krak bezi implantatsiyasiga ega 173 ayol ko'krakning ALCLini ishlab chiqdilar. Ular "Ko'krak implantatsiyasi bilan bog'liq bo'lgan ALCL - bu turli xil xususiyatlarni aks ettiruvchi va multifaktorial sababni ko'rsatadigan, ma'lum bir chandiq joyidan kelib chiqqan joy va materialga xos lenfomaning yangi namoyonidir" degan xulosaga kelishdi. Ular "fiziologik eritma yoki silikon plomba moddasi yoki kosmetik yoki rekonstruktiv ko'rsatkichlar uchun hech qanday afzallik yo'qligini" ta'kidladilar. Implantatsiya tarixi ma'lum bo'lgan joyda, bemorga kamida bitta tekstura qilingan uskuna berilgan. 2016 yilda Jahon sog'liqni saqlash tashkiloti (JSST) rasmiy ravishda tan olingan BIA-ALCL.[71]
2017 yil 1-fevral holatiga ko'ra FDA ko'krak bezi implantatsiyasiga aloqador ALCL (BIALCL) haqida jami 359 ta tibbiy asbob-uskunalar haqida hisobot oldi, shu jumladan 9 ta o'lim.[72] Ko'krak implantlari va ALCL o'rtasidagi nedensel assotsiatsiya 2013 yil dekabr oyida, MD Anderson saraton markazining tadqiqotchilari, ko'krakda ALCL tashxisi qo'yilgan 60 nafar ko'krak implantlari bo'lgan ayollarning tadqiqotlarini e'lon qilishganda aniqlandi. ALCL yarim milliondan atigi 1 ayolda aniqlanadi deb o'ylaganligi sababli, 60 ayol kutilganidan ancha yuqori edi. Tadqiqotchilar BIA-ALCL o'limga olib kelishi mumkinligini ta'kidladilar.[73] Agar implantatsiyaga ega ayollar kechiktirilgan shish yoki suyuqlik to'planishi bilan murojaat qilsalar, sitologik tadqiqotlar va marker uchun test CD30 taklif qilinmoqda. Amerika Plastik Jarrohlik Jamiyati (ASPS) ta'kidlashicha, "CD30 - bu odatdagi patologiya yoki seroma suyuqligi bo'yicha o'tkazilishi kerak bo'lgan asosiy diagnostik test. H&E binoni tez-tez tashxisni o'tkazib yuborishi mumkin. " [74] Ko'krak implantlari bilan bog'liq bo'lgan ALCL diagnostikasi va davolash endi Milliy keng qamrovli saraton tarmog'i tomonidan belgilangan standart ko'rsatmalarga amal qiladi.[75]
AQShda mavjud bo'lgan BIA-ALCL xavfi noma'lum, ammo taxminlarga ko'ra, ko'krak implantlari bilan kasallangan ayollarning 70,000 dan 500,000tasi orasida. MD Anderson saraton markazi.[76] Muayyan geografik joylar o'zgaruvchan xatarlarni namoyish etdi. Masalan, 2016 yil dekabr oyida Avstraliya va Yangi Zelandiyaning Terapevtik tovarlar ma'muriyatining yangilanishida teksturali implantatlar uchun 1: 1000 dan 1: 10,000 gacha bo'lgan xavf haqida xabar berilgan. "[74] Bugungi kunga qadar (2017), bemorda faqat silliq qobiq ko'krak implantatsiyasini yoki silliq implantga almashtirilgan tekstura qilingan to'qima kengaytiruvchisini implantatsiyasi bo'lgan BIAL holati bo'lmagan. Osiyo populyatsiyalarida qayd etilgan holatlarning kamligi bu hodisaga genetik ta'sirchanlik ehtimoli borligini oshirdi; Shu bilan bir qatorda, u holatlarni aniqlash va xabar berishdagi farqlarni aks ettirishi mumkin.
ASPS va Plastik Jarrohlik Jamg'armasi (PSF) FDA bilan hamkorlikda ushbu holatni o'rganishdi va shu bilan Bemorni ro'yxatga olish kitobi va ko'krak implantlari va anaplastik katta hujayrali limfoma etiologiyasi va epidemiologiyasi natijalarini (PROFIL) yaratdilar. Amerika Qo'shma Shtatlari FDA barcha implantlarni ALCLda ko'krak implantlarining rolini va ushbu kasallikni boshqarishni yaxshiroq anglash maqsadida PROFILE-ga xabar berishlarini qat'iyan tavsiya qiladi.[77]
Jarrohlik muolajalari
Kesish turlari
Ko'krak implantatsiyasining joylashishi besh (5) turdagi jarrohlik kesmalar bilan amalga oshiriladi:
- Inframammary: kesma inframammar katlama (ko'krak ostidagi tabiiy burish), bu to'qimalarni aniq ajratish va ko'krak implantlarini joylashtirish uchun maksimal imkoniyatni beradi. Bu silikon-gel implantlarini joylashtirish uchun afzal qilingan jarrohlik texnikasi, chunki u ta'sirini yaxshiroq ta'sir qiladi ko'krak to'qimasi –ko'krak mushaklari interfeys; Hali ham IMF implantatsiyasi qalinroq va biroz ko'rinadigan jarrohlik operatsiyasini amalga oshirishi mumkin chandiqlar.
- Periareolar: ning atrofi bo'ylab chegara chizig'i kesmasi areola, bu XVF pozitsiyasiga o'zgartirishlar kiritish zarur bo'lganda yoki a mastopeksiya (ko'krakni ko'tarish) asosiy mammoplastika protsedurasiga kiritilgan. Periareolar joylashuvida kesma areola atrofining medial yarmi (pastki yarmi) atrofida bo'ladi. Silikon jel implantlarini periareolar kesma orqali bo'shatish qiyin bo'lishi mumkin, chunki kerakli kirish kesmasining qisqa, besh santimetr uzunligi (~ 5,0 sm). Estetik jihatdan, chandiqlar izolaning chegarasida (periferiya) bo'lganligi sababli, ular odatda engil pigmentli areolali ayollarning XVF tomonidan kesilgan izlariga qaraganda kamroq ko'rinadi; teri-kesma izlari bilan taqqoslaganda, o'zgartirilgan epiteliya gipertrofik chandiqlarga kamroq moyil bo'lgan (ko'tarilgan).
- Transaksiller: kesma qo'ltiq osti (qo'ltiq osti), bu orqali implantlarni implantatsiyani to'g'ridan-to'g'ri yoki bo'shliq bilan almashtirish uchun medial ravishda tunnellar endoskop (yoritilgan videokamera), ko'krakda ko'rinadigan chandiqlar paydo bo'lmasdan; Shunga qaramay, implant-moslama pozitsiyasining past assimetriyasini hosil qilish osonroq. Shu sababli, transaksillarar joylashtirilgan ko'krak implantlarini jarrohlik yo'li bilan qayta ko'rib chiqish uchun odatda XVF kesmasi yoki periareolar kesma kerak.
- Transumbilikal: ko'krak qafasining trans-kattalashishi (TUBA ) - bu implant-qurilmani joylashtirish usuli, bu kesma kindik qismida joylashgan (kindik ) va disektsiya tunnellari ustuniga, yuqoriga qarab. TUBA yondashuvi ko'krak qafasida aniq jarohatlar paydo bo'lmasdan, ko'krak implantlarini joylashtirishga imkon beradi; ammo mos ravishda ajratish va moslamalarni joylashtirishni texnik jihatdan qiyinlashtiradi. TUBA protsedurasi endoskopning ko'rgazmali yordamisiz aniq bajariladi va silikon-gel implantlarini joylashtirish uchun mos emas, chunki bu shikastlanish uchun katta imkoniyatlarga ega. elastomer silikon ko'krak implantatsiyasining qobig'i, uni kindik qismidagi qisqa (~ 2,0 sm) kesma orqali qo'lda kiritish paytida va oldindan to'ldirilgan silikon jel implantlari siqilmasligi sababli juda kichik kesma orqali kiritib bo'lmaydi.[78]
- Transabdominal: TUBA protsedurasida bo'lgani kabi, transabdominoplastikada ko'krakni kattalashtirishda (TABA), ko'krak implantlari qorin kesimidan to'g'ridan-to'g'ri kesilgan implant cho'ntaklariga tunnel qilinadi, bemor esa bir vaqtning o'zida Abdominoplastika.[79]
Implantatsiya cho'ntagini joylashtirish
Besh jarrohlik ko'krak implantatsiyasini implant cho'ntagiga joylashtirish yondashuvlari ko'pincha tavsiflanadi anatomik ga munosabat katta mushak.
- Subglandular: ko'krak implantatsiyasi joylashtirilgan retromammar bo'shliq, o'rtasida ko'krak to'qimasi (sut bezlari) va katta mushak (ko'krak qafasining asosiy mushaklari), bu oddiy ko'krak to'qimalarining tekisligiga yaqinlashadi va eng estetik natijalarni beradi. Shunga qaramay, ingichka pektoral yumshoq to'qimalarga ega ayollarda subglandular pozitsiya asosiy implantning to'lqinlari va ajinlarini ko'rsatishi mumkin. Bundan tashqari, kapsula kontrakturasi subglandular implantatsiya bilan kasallanish darajasi biroz kattaroq.
- Subfasiyal: ko'krak implantatsiyasi ostiga joylashtirilgan fasya ning katta mushak; subfasiyal pozitsiya ko'krak implantatsiyasi uchun subglandular pozitsiyaning bir variantidir.[80] Subfasiyal implantatsiya-cho'ntak texnikasining texnik afzalliklari muhokama qilinadi; tarafdor jarrohlarning ta'kidlashicha, qatlami fasial to'qima ko'proq implantatsiyani qamrab oladi va o'z mavqeini yaxshilaydi.[81]
- Subpektoral (ikki tomonlama tekislik): ko'krak implantatsiyasi ostiga joylashtirilgan katta mushak, jarroh pastki mushak qo'shimchalarini chiqargandan so'ng, subglandular tekislikni qisman ajratish bilan yoki bo'lmasdan. Natijada, implantning yuqori qutbi qisman katta pektoral mushak ostida, implantning pastki qutbasi esa subglandular tekislikda joylashgan. Ushbu implantatsiya texnikasi implantning yuqori qutbini maksimal darajada qoplashga imkon beradi, shu bilan birga implantning pastki qutbini kengaytirishga imkon beradi; ammo, "animatsiya deformatsiyasi", implantlarning subpektoral tekislikda harakatlanishi ba'zi bemorlar uchun haddan tashqari ko'p bo'lishi mumkin.[82]
- Submuskulyar: ko'krak osti implantatsiyasi ostiga joylashtirilgan katta mushak, mushakning pastki kelib chiqishini to'g'ri chiqarmasdan. Implantatsiyani mushaklarning to'liq qamrab olishiga ko'krak devorining lateral mushaklarini bo'shatish orqali erishish mumkin - yoki serratus mushak yoki pektoralis kichik mushak yoki ikkalasi ham va tikish u yoki ular, katta ko'krak mushagiga. Yilda ko'krakni qayta tiklash jarrohlik, submuskular implantatsiya yondashuvi ko'krak implantlarini maksimal darajada qamrab olishiga ta'sir qiladi. Ushbu uslub kosmetik jarrohlikda animatsiya deformatsiyalari xavfi yuqori bo'lganligi sababli kamdan kam qo'llaniladi.
- Prepektoral yoki teri osti: terini tejash yoki terini va nipelni saqlovchi mastektomiyadan so'ng ko'krakni qayta tiklashda implant yuqoridan qo'yiladi pektoralis major implantni olib tashlangan holda to'g'ridan-to'g'ri sut bezini to'ldirishi uchun uni ajratmasdan mushak. Kapsül kontrakturası muammosini oldini olish uchun, implantatsiya ko'pincha oldingi yoki to'liq qopqoq bilan qoplanadi biomaterial biologik yoki sintetik.
Jarrohlikdan keyingi tiklanish
The jarrohlik chandiqlar a ko'krakni kattalashtirish mammoplastika operatsiyadan keyingi 6 xaftada rivojlanadi va bir necha oy ichida susayadi. Ayol uchun zarur bo'lgan kundalik hayotiy jismoniy faoliyatga qarab, ko'krak kattalashtiruvchi bemor odatda operatsiyadan keyingi 1 xaftada normal hayotini davom ettiradi. Bundan tashqari, ko'krak qafasi implantatsiyalari ko'krak mushaklari ostiga joylashtirilgan (mushak osti mushaklarining joylashishi) ayollarda, odatda, ko'krak mushaklaridagi kesmalarning davolanishi tufayli biroz uzoqroq og'riqli tuzalish kuzatiladi. Odatda, u taxminan 6 hafta davomida jismoniy mashqlar qilmaydi yoki og'ir jismoniy mashqlar bilan shug'ullanmaydi. Operatsiyadan keyingi dastlabki tiklanish paytida ayol og'riq va bezovtalikni yumshatish uchun qo'lini muntazam ravishda mashq qilishi (egilishi va harakatlanishi) tavsiya etiladi; agar kerak bo'lsa, og'riq qoldiruvchi yashash kateterlari og'riqni engillashtirishi mumkin[83][84] Bundan tashqari, bemorlarning tiklanishi sezilarli darajada yaxshilandi, bu ayollarning 95 foiziga protseduradan keyingi 24 soat davomida bintlarsiz, suyuqlikni to'kib tashlaydigan vositalarsiz, og'riq qoldiruvchi nasoslar va kateterlarsiz normal hayotini tiklashga imkon beradigan ko'krak qafasi apparati implantatsiyasining takomillashtirilgan usullari (submuskular, subglandular). , tibbiy yordam brassierlari yoki giyohvandlik og'riq qoldiruvchi dorilar.[85][86][87][88]
Turlari

Bugungi kunda, odatda ishlatiladigan uchta turdagi implantlar mavjud mammaplastika, ko'krakni qayta tiklash va ko'krakni kattalashtirish protseduralar:[89]
- steril bilan to'ldirilgan fiziologik eritma fiziologik eritma.
- yopishqoq bilan to'ldirilgan silikon implant silikon jel.
- ichki elastomer silikon chig'anoqlari va ikkita sho'r suv bilan to'ldirilgan lümen yordamida tuzilgan implantlar.
Implantlarning to'rtinchi turi, kompozit (yoki muqobil-kompozit) implantatlar, asosan, to'xtatildi. Ushbu turdagi soya yog'i va polipropilen ip kabi plomba moddalari mavjud. Boshqa to'xtatilgan materiallar orasida ho'kiz xaftaga, terilen yünü, maydalangan kauchuk, silastik kauchuk va teflon-silikon protezlar mavjud.[90]
Tuzli implantlar
Tuzli ko'krak implantatsiyasi to'ldirilgan fiziologik eritma (biologik konsentratsiyali sho'r suv 0,90% w / v ning NaCl, taxminan 300 mOsm /L.)— birinchi bo'lib Laboratoires Arion kompaniyasi tomonidan Frantsiyada ishlab chiqarilgan va protez sifatida foydalanish uchun kiritilgan tibbiy asbob 1964 yilda. Tuzli ko'krak implantatsiyasining zamonaviy modellari xona harorati qalinroq ishlab chiqarilgan vulkanizatsiya qilingan (RTV) dan yasalgan snaryadlar silikon elastomer. O'qish Oldindan to'ldirilgan sho'rlangan ko'krak implantlarining in vitro deflyatsiyasi (2006) oldindan to'ldirilgan sho'rlangan ko'krak implantatsiyasining deflyatsiya darajasi (plomba oqishi) uni ko'krak tuzatish operatsiyasi uchun ikkinchi tanlov proteziga aylantirganligi haqida xabar bergan.[91] Shunga qaramay, 1990-yillarda sho'rlangan ko'krak implantatsiyasi bu edi protez most common device used for breast augmentation surgery in the United States, because of the U.S. FDA's restriction against the implantation of silicone-filled breast implants outside of clinical studies. Saline breast implants have enjoyed little popularity in the rest of the world, possessing negligible market share.
The technical goal of saline-implant technology was a physically less invasive surgical technique for emplacing an empty breast implant device through a smaller surgical incision.[92] In surgical praxis, after having emplaced the empty breast implants to the implant pockets, the plastic surgeon then filled each device with fiziologik eritma, and, because the required insertion-incisions are short and small, the resultant incision-scars will be smaller and shorter than the surgical scars usual to the long incisions required for inserting pre-filled, silicone-gel implants.
When compared to the results achieved with a silicone-gel breast implant, the saline implant can yield acceptable results, of increased breast-size, smoother hemisphere-contour, and realistic texture; yet, it is likelier to cause cosmetic problems, such as the rippling and the wrinkling of the breast-envelope skin, accelerated lower breast pole stretch, and technical problems, such as the presence of the implant being noticeable to the eye and to the touch. The occurrence of such cosmetic problems is likelier in the case of the woman with very little ko'krak tissue, and in the case of the woman who requires post-mastectomy breast reconstruction; thus, the silicone-gel implant is the technically superior protez device for breast augmentation, and for ko'krakni qayta tiklash. In the case of the woman with much breast tissue, for whom sub-muscular emplacement is the recommended surgical approach, saline breast implants can produce an aesthetic result much like that afforded by silicone breast implants, albeit with greater implant palpability.[93]
Silicone gel implants
Kabi tibbiy asbob texnologiya, there are five generations of silicone breast implant, each defined by common model-manufacturing techniques.[iqtibos kerak ]
The modern prosthetic breast was invented in 1961 by the American plastik jarrohlar Thomas Cronin and Frank Gerow, and manufactured by the Dow Corning korporatsiyasi; in due course, the first augmentation mammoplasty was performed in 1962.
Birinchi avlod
The Cronin–Gerow Implant, prosthesis model 1963, was a silicone rubber envelope-sac, shaped like a teardrop, which was filled with viscous silicone-gel. To reduce the rotation of the emplaced breast implant upon the chest wall, the model 1963 prosthesis was affixed to the implant pocket with a fastener-patch, made of Dacron material (Polietilen tereftalat ), which was attached to the rear of the breast implant shell.[94]
Ikkinchi avlod
In the 1970s, manufacturers presented the second generation of breast implant prostheses that featured functional developments and aesthetic improvements to the technology:
- the first technological developments were a thinner-gauge device-shell, and a filler gel of low-cohesion silicone, which improved the functionality and the verisimilitude (size, appearance, and texture) of the silicone-gel breast implant. Yet, in clinical practice, second-generation breast implants proved fragile, and suffered greater incidences of shell rupture, and of filler leakage ("silicone-gel bleed") through the intact device shell. The consequent, increased incidence-rates of medical complications (e.g. kapsula kontrakturasi ) precipitated faulty-product, class action-lawsuits, by the U.S. government, against the Dow Corning Corporation, and other manufacturers of breast prostheses.
- the second technological development was a ko'pikli poliuretan coating for the shell of the breast implant; the coating reduced the incidence of kapsula kontrakturasi, by causing an yallig'lanish reaktsiyasi that impeded the formation of a capsule of fibrous kollagen tissue around the breast implant. Nevertheless, despite that prophylactic measure, the medical use of polyurethane-coated breast implants was briefly discontinued, because of the potential health-risk posed by 2,4-toluenediamine (TDA), a kanserogen by-product of the chemical breakdown of the polyurethane foam coating of the breast implant.[95]
- After reviewing the medical data, the U.S. Oziq-ovqat va dori-darmonlarni boshqarish concluded that TDA-induced ko'krak bezi saratoni was an infinitesimal health-risk to women with breast implants, and did not justify legally requiring physicians to explain the matter to their patients. In the event, polyurethane-coated breast implants remain in plastic surgery practice in Europe and in South America; and no manufacturer has sought FDA approval for medical sales of such breast implants in the U.S.[96]
- the third technological development was the double lumen breast implant device, a double-cavity prosthesis composed of a silicone breast implant contained within a saline breast implant. The two-fold, technical goal was: (i) the cosmetic benefits of silicone-gel (the inner lumen) enclosed in saline solution (the outer lumen); (ii) a breast implant device the volume of which is post-operatively adjustable. Nevertheless, the more complex design of the double-lumen breast implant suffered a device-failure rate greater than that of single-lumen breast implants. The contemporary versions of second-generation breast implant devices (presented in 1984) are the "Becker Expandable" models of breast implant, which are primarily used for ko'krakni qayta tiklash.
Third and Fourth generations
In the 1980s, the models of the Third and of the Fourth generations of breast implant devices were sequential advances in manufacturing technology, such as elastomer -coated shells that decreased gel-bleed (filler leakage), and a thicker (increased-cohesion) filler gel. Sociologically, the manufacturers of prosthetic breasts then designed and made anatomic models (natural breast) and shaped models (round, tapered) that realistically corresponded with the breast- and body- types of women. The tapered models of breast implant have a uniformly textured surface, which reduces the rotation of the prosthesis within the implant pocket; the round models of breast implant are available in smooth-surface- and textured-surface- types.
Beshinchi avlod
Since the mid-1990s, the fifth generation of silicone-gel breast implant is made of a high-strength, highly cohesive silicone gel that mostly eliminates the occurrences of filler leakage (“silicone gel bleed”) and of the migration of the silicone filler from the implant pocket to elsewhere in the woman's body. These implants are commonly referred to as "gummy bear breast implants" for their firm, pliant consistency, which is similar to gummy candies. The studies Experience with Anatomical Soft Cohesive Silicone gel Prosthesis in Cosmetic and Reconstructive Breast Implant Surgery (2004) va Cohesive Silicone gel Breast Implants in Aesthetic and Reconstructive Breast Surgery (2005) reported low incidence-rates of kapsula kontrakturasi and of device-shell rupture; and greater rates of improved medical-safety and technical-efficacy than that of early generation breast implant devices.[97][98][99]
Structured implants
Structured implants were approved by the FDA and Health Canada in 2014 as a third form of breast implant.[100] Structured implants incorporate both saline and silicone gel implant technology. The filler is only saline solution in case of rupture and has a natural feel like silicone gel implants.[101] The implant uses an internal structure which consists of a series of nested shells that support the upper pole with the two lumen being filled with only saline. The implant is inserted empty and then filled once in place which requires less of an incision than pre-filled implants.[100] If one of the lumen of the structured implant ruptures, it leaks and empties. The other lumen remain intact and the implant only partially deflates, allowing for ease of explant and replacement.[100]
Ko'krak suti bilan boqish
The presence of breast implants currently presents no contraindication to breast feeding, and no evidence to support that the practice may present health issues to a breast feeding infant is recognized by the USFDA.
Women with breast implants may have functional breast-feeding difficulties; mammoplasty procedures that feature periareolar incisions are especially likely to cause breast-feeding difficulties. Surgery may also damage the lactiferous ducts and the nerves in the nipple-areola area.[102][103][104]
Functional breast-feeding difficulties arise if the surgeon cut the milk ducts or the major nerves innervating the breast, or if the milk glands were otherwise damaged. Milk duct and nerve damage are more common if the incisions cut tissue near the nipple. The milk glands are most likely to be affected by subglandular implants (under the gland), and by large-sized breast implants, which pinch the lactiferous ducts and impede milk flow. Small-sized breast implants, and submuscular implantation, cause fewer breast-function problems; however, it is impossible to predict whether a woman who undergoes breast augmentation will be able to successfully breast feed since some women are able to breast-feed after periareolar incisions and subglandular placement and some are not able to after augmentation using submuscular and other types of surgical incisions.[104]
Mamografi

Mavjudligi radiologically opaque breast implants (either saline or silicone) might interfere with the radiographic sensitivity of the mamograf, that is, the image might not show any tumor(s) present. In this case, an Eklund view mammogram is required to ascertain either the presence or the absence of a cancerous tumor, wherein the breast implant is manually displaced against the chest wall and the breast is pulled forward, so that the mammograph can visualize a greater volume of the internal tissues; nonetheless, approximately one-third of the breast tissue remains inadequately visualized, resulting in an increased incidence of mammograms with false-negative results.[105][106]
The ko'krak bezi saratoni tadqiqotlar Cancer in the Augmented Breast: Diagnosis and Prognosis (1993) va Breast Cancer after Augmentation Mammoplasty (2001) of women with breast implant prostheses reported no significant differences in disease-stage at the time of the diagnosis of cancer; prognoses are similar in both groups of women, with augmented patients at a lower risk for subsequent cancer recurrence or death.[107][108] Conversely, the use of implants for ko'krakni qayta tiklash keyin ko'krak bezi saratoni mastektomiya appears to have no negative effect upon the incidence of cancer-related death.[109] That patients with breast implants are more often diagnosed with palpable—but not larger—tumors indicates that equal-sized tumors might be more readily palpated in augmented patients, which might compensate for the impaired mammogram images.[110] The ready palpability of the breast-cancer tumor(s) is consequent to breast tissue thinning by compression, innately in smaller breasts apriori (because they have lesser tissue volumes), and that the implant serves as a radio-opaque base against which a cancerous tumor can be differentiated.[111]
The breast implant has no clinical bearing upon lumpektomiya breast-conservation surgery for women who developed breast cancer after the implantation procedure, nor does the breast implant interfere with external beam radiation treatments (XRT); moreover, the post-treatment incidence of breast-tissue fibrosis is common, and thus a consequent increased rate of capsular kontraktura.[112] There is tentative evidence that women who have had breast augmentation, have worse breast cancer prognosis.[113] The use of implants for ko'krakni qayta tiklash keyin ko'krak bezi saratoni mastektomiya appears to have no negative effect upon cancer-related death.[109][114]
Tarix

19-asr
Since the late nineteenth century, breast implants have been used to jarrohlik yo'li bilan augment the size (volume), modify the shape (contour), and enhance the feel (tact) of a woman's breasts. In 1895, surgeon Vincenz Czerny effected the earliest breast implant emplacement when he used the patient's autologous yog 'to'qimasi, harvested from a benign bel lipoma, to repair the asymmetry of the breast from which he had removed a tumor.[115] In 1889, surgeon Robert Gersuny experimented with kerosin injections, with disastrous results arising from the breakup of the paraffin into smaller bodies following the procedure.[116]
20-asr
From the first half of the twentieth century, physicians used other substances as breast implant fillers—fil suyagi, glass balls, ground kauchuk, ho'kiz xaftaga, Terilen jun, gutta-percha, Dicora, polietilen chips, Ivalon (polivinil spirt —formaldehyde polymer sponge), a polyethylene sac with Ivalon, polyether foam sponge (Etheron), polyethylene tape (Polystan) strips wound into a ball, polyester (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.[117]
In the mid-twentieth century, Morton I. Berson, in 1945, and Jacques Maliniac, in 1950, each performed flap-based breast augmentations by rotating the patient's chest wall tissue into the breast to increase its volume. Furthermore, throughout the 1950s and the 1960s, plastic surgeons used synthetic fillers—including silikon injections received by some 50,000 women, from which developed silicone granulomalar and breast hardening that required treatment by mastektomiya.[118] In 1961, the American plastic surgeons Thomas Cronin and Frank Gerow, and the Dow Corning korporatsiyasi, developed the first silicone breast prosthesis, filled with silicone gel; in due course, the first augmentation mammoplasty was performed in 1962 using the Cronin–Gerow Implant, prosthesis model 1963. In 1964, the French company Laboratoires Arion developed and manufactured the saline breast implant, filled with fiziologik eritma, and then introduced for use as a tibbiy asbob 1964 yilda.[91]
FDA tomonidan tasdiqlangan
In 1988, twenty-six years after the 1962 introduction of breast implants filled with silicone gel, the AQSh oziq-ovqat va farmatsevtika idorasi (FDA) investigated breast implant failures and the subsequent asoratlar, and re-classified breast implant devices as Class III medical devices, and required from manufacturers the documentary data substantiating the safety and efficacy of their breast implant devices.[119] In 1992, the FDA placed silicone-gel breast implants in moratorium in the U.S., because there was “inadequate information to demonstrate that breast implants were safe and effective”. Nonetheless, medical access to silicone-gel breast implant devices continued for clinical studies of post-mastectomy ko'krakni qayta tiklash, the correction of congenital deformities, and the replacement of ruptured silicone-gel implants. The FDA required from the manufacturers the clinical trial data, and permitted their providing breast implants to the ko'krakni kattalashtirish patients for the statistical studies required by the U.S. Food and Drug Administration.[119] In mid–1992, the FDA approved an adjunct study protocol for silicone-gel filled implants for breast reconstruction patients, and for revision-surgery patients. Shuningdek, 1992 yilda Dow Corning korporatsiyasi, a silicone products and breast implant manufacturer, announced the discontinuation of five implant-grade silikonlar, but would continue producing 45 other, medical-grade, silicone materials—three years later, in 1995, the Dow Corning Corporation went bankrot when it faced large class action lawsuits claiming a variety of illnesses.[119]
- 1997 yilda, AQSh Sog'liqni saqlash va aholiga xizmat ko'rsatish vazirligi (HHS) appointed the Institute of Medicine (IOM) of the AQSh Milliy Fanlar Akademiyasi (NAS) to investigate the potential risks of operative and post-operative asoratlar from the emplacement of silicone breast implants. The IOM's review of the safety and efficacy of silicone gel-filled breast implants, reported that the "evidence suggests diseases or conditions, such as biriktiruvchi to'qima kasalliklar, saraton, neurological diseases, or other systemic complaints or conditions are no more common in women with breast implants, than in women without implants" subsequent studies and systemic review found no causal link between silicone breast implants and disease.[119]

- In 1998, the U.S. FDA approved adjunct study protocols for silicone-gel filled implants only for breast reconstruction patients and for revision-surgery patients; and also approved the Dow Corning Corporation's Investigational Device Exemption (IDE) study for silicone-gel breast implants for a limited number of breast augmentation-, reconstruction-, and revision-surgery patients.[119]
- In 1999, the Institute of Medicine published the Safety of Silicone Breast Implants (1999) study that reported no evidence that saline-filled and silicone-gel filled breast implant devices caused systemic health problems; that their use posed no new health or safety risks; and that local complications are “the primary safety issue with silicone breast implants”, in distinguishing among routine and local medical complications and systemic health concerns.”[119][120][121]
- In 2000, the FDA approved saline breast implant Premarket Approval Applications (PMA) containing the type and rate data of the local medical complications experienced by the breast surgery patients.[122] "Despite complications experienced by some women, the majority of those women still in the Inamed Corporation va Mentor Corporation studies, after three years, reported being satisfied with their implants."[119] The premarket approvals were granted for breast augmentation, for women at least 18 years old, and for women requiring ko'krakni qayta tiklash.[123][124]
- In 2006, for the Inamed Corporation and for the Mentor Corporation, the U.S. Food and Drug Administration lifted its restrictions against using silicone-gel breast implants for breast reconstruction and for augmentation mammoplasty. Yet, the approval was conditional upon accepting FDA monitoring, the completion of 10-year-mark studies of the women who already had the breast implants, and the completion of a second, 10-year-mark study of the safety of the breast implants in 40,000 other women.[125] The FDA warned the public that breast implants do carry medical risks, and recommended that women who undergo ko'krakni kattalashtirish should periodically undergo MRI examinations to screen for signs of either shell rupture or of filler leakage, or both conditions; and ordered that breast surgery patients be provided with detailed, informational brochures explaining the medical risks of using silicone-gel breast implants.[119]
The U.S. Food and Drug Administration established the age ranges for women seeking breast implants; for breast reconstruction, silicone-gel filled implants and saline-filled implants were approved for women of all ages; for breast augmentation, saline implants were approved for women 18 years of age and older; silicone implants were approved for women 22 years of age and older.[126] Because each breast implant device entails different medical risks, the minimum age of the patient for saline breast implants is different from the minimum age of the patient for silicone breast implants—because of the filler leakage and silent shell-rupture risks; thus, periodic MRI screening examinations are the recommended post-operative, follow-up therapy for the patient.[126] In other countries, in Europe and Oceania, the national health ministries' breast implant policies do not endorse periodic MRI screening of asymptomatic patients, but suggest palpation proper—with or without an ultratovushli screening—to be sufficient post-operative therapy for most patients.
Shuningdek qarang
Adabiyotlar
- ^ "Risks and Complications of Breast Implants". FDA. 21 oktyabr 2019 yil. Olingan 30 oktyabr 2019.
- ^ Amerika plastik jarrohlar jamiyati (2014 yil 24-aprel), "Shifokorlar va bemorlar so'rashlari kerak bo'lgan beshta narsa", Aql bilan tanlash: ning tashabbusi ABIM Foundation, American Society of Plastic Surgeons, archived from asl nusxasi 2014 yil 19-iyulda, olingan 25 iyul 2014
- ^ Brinton LA, Brown SL, Colton T, Burich MC, Lubin J (2000). "Characteristics of a Population of Women with Breast Implants Compared with Women Seeking other Types of Plastic Surgery". Plastik va rekonstruktiv jarrohlik. 105 (3): 919–927. doi:10.1097/00006534-200003000-00014. PMID 10724251. S2CID 32599107.
- ^ Jacobsen PH, Hölmich LR, McLaughlin JK, Johansen C, Olsen JH, Kjøller K, Friis S (2004). "Mortality and suicide among Danish women with cosmetic breast implants". Arch. Stajyor. Med. 164 (22): 2450–5. doi:10.1001/archinte.164.22.2450. PMID 15596635.
- ^ Young VL, Nemecek JR, Nemecek DA (1994). "The Efficacy of Breast Augmentation: Breast Size Increase, Patient Satisfaction, and Psychological Effects". Plastik va rekonstruktiv jarrohlik. 94 (Dec): 958–969. doi:10.1097/00006534-199412000-00009. PMID 7972484. S2CID 753343.
- ^ Crerand CE, Franklin ME, Sarwer DB (2006). "Body Dysmorphic Disorder and Cosmetic Surgery". Plastik va rekonstruktiv jarrohlik. 118 (July): 167e–180e. doi:10.1097/01.prs.0000242500.28431.24. PMID 17102719. S2CID 8925060.
- ^ Sarwer DB, LaRossa D, Bartlett SP, Low DW, Bucky LP, Whitaker LA (2003). "Body Image Concerns of Breast Augmentation Patients". Plastik va rekonstruktiv jarrohlik. 112 (July): 83–90. doi:10.1097/01.PRS.0000066005.07796.51. PMID 12832880. S2CID 45574374.
- ^ Chahraoui K, Danino A, Frachebois C, Clerc AS, Malka G (2006). "Aesthetic Surgery and Quality of Life Before and Four Months Postoperatively". Journal of Long-Term Effects of Medical Implants. 51 (3): 207–210. doi:10.1016/j.anplas.2005.07.010. PMID 16181718.
- ^ Cash TF, Duel LA, Perkins LL (2002). "Women's Psychosocial Outcomes of Breast Augmentation with Silicone gel-filled implants: a 2-year Prospective Study". Plastik va rekonstruktiv jarrohlik. 109 (May): 2112–2121. doi:10.1097/00006534-200205000-00049. PMID 11994621.
- ^ Figueroa-Haas CL (2007). "Effect of Breast Augmentation Mammoplasty on Self-esteem and Sexuality: A Quantitative Analysis". Plastic Surgery Nursing. 27 (Mar): 16–36. doi:10.1097/01.PSN.0000264159.30505.c9. PMID 17356451. S2CID 23169107.
- ^ "Important Information for Women About Breast Augmentation with Inamed Silicone Gel-Filled Implants" (PDF). 2006. Arxivlangan asl nusxasi (PDF) 2007-01-03 da. Iqtibos jurnali talab qiladi
| jurnal =(Yordam bering) - ^ Handel N, Cordray T, Gutierrez J, Jensen JA (2006). "A Long-term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants". Plastik va rekonstruktiv jarrohlik. 117 (Mar): 757–767. doi:10.1097 / 01.prs.0000201457.00772.1d. PMID 16525261. S2CID 15228702.
- ^ "Breast Implants Linked with Suicide in Study". Reuters. 2007-08-08. Arxivlandi from the original on 2008-12-21.
- ^ Manning A (2007-08-06). "Breast Implants Linked to Higher Suicide Rates". USA Today. Arxivlandi from the original on 2011-03-18. Olingan 2010-04-26.
- ^ Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN (2001). "Cancer risk at sites other than the breast following augmentation mammoplasty". Ann Epidemiol. 11 (4): 248–56. doi:10.1016/s1047-2797(00)00223-4. PMID 11306343.
- ^ Koot VC, Peeters PH, Granath F, Grobbee DE, Nyren O (2003). "Total and cause specific mortality among Swedish women with cosmetic breast implants: prospective study". BMJ. 326 (7388): 527–8. doi:10.1136/bmj.326.7388.527. PMC 150462. PMID 12623911.
- ^ Pukkala E, Kulmala I, Hovi SL, Hemminki E, Keskimäki I, Pakkanen M, Lipworth L, Boice JD, McLaughlin JK (2003). "Causes of death among Finnish women with cosmetic breast implants, 1971-2001". Ann Plast Surg. 51 (4): 339–42, discussion 343–4. doi:10.1097/01.sap.0000080407.97677.A5. PMID 14520056. S2CID 34929987.
- ^ Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y (2006). "Mortality among Canadian women with cosmetic breast implants". Am. J. Epidemiol. 164 (4): 334–41. doi:10.1093/aje/kwj214. PMID 16777929.
- ^ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality rates among augmentation mammoplasty patients: an update". Epidemiologiya. 17 (2): 162–9. doi:10.1097/01.ede.0000197056.84629.19. PMID 16477256. S2CID 22285852.
- ^ National Plastic Surgery Procedural Statistics, 2006. Arlington Heights, Illinois, American Society of Plastic Surgeons, 2007
- ^ "Plastic Surgery Helps Self-Esteem". Psych Central.com. Arxivlandi asl nusxasidan 2010-06-19.
- ^ Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F (2013). "Late unilateral hematoma after breast reconstruction with implants: case report and literature review". Aesthetic Surgical Journal. 33 (6): 830–834. doi:10.1177/1090820X13496249. PMID 23864111.
- ^ a b "Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants" (PDF). 2006-11-03. Arxivlandi asl nusxasi (PDF) 2007-01-03 da. Olingan 2007-05-04.
- ^ "Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants" (PDF). 2006-11-03. Arxivlandi asl nusxasi (PDF) 2014 yil 16 oktyabrda. Olingan 11 oktyabr 2014.
- ^ "Saline-Filled Breast Implant Surgery: Making An Informed Decision (Mentor Corporation)". FDA Breast Implant Consumer Handbook - 2004. 2004-01-13. Arxivlandi asl nusxasi 2006-11-26 kunlari. Olingan 2007-05-04.
- ^ "FDA NEWS RELEASE". Arxivlandi asl nusxadan 2011-11-03. Olingan 2011-11-09.
- ^ Breiting VB, Hölmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjøller K, McLaughlin JK, Wiik A, Friis S (2004). "Long-term Health Status of Danish Women with Silicone Breast Implants". Plastik va rekonstruktiv jarrohlik. 114 (1): 217–226. doi:10.1097/01.PRS.0000128823.77637.8A. PMID 15220596. S2CID 20584928.
- ^ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality Rates Among Augmentation Mammoplasty Patients: An Update". Epidemiologiya. 17 (2): 162–9. doi:10.1097/01.ede.0000197056.84629.19. PMID 16477256. S2CID 22285852.
- ^ Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y (June 2006). "Mortality Among Canadian Women with Cosmetic Breast Implants". Amerika Epidemiologiya jurnali. 164 (4): 334–341. doi:10.1093/aje/kwj214. PMID 16777929.
- ^ Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL (1996). "Breast Enlargement and Reduction: Results from a Breast Cancer Case-control Study". Plastik va rekonstruktiv jarrohlik. 97 (2): 269–275. doi:10.1097/00006534-199602000-00001. PMID 8559808. S2CID 29456173.
- ^ Benadiba L (2004). "Histoire des protheses mammaires" (frantsuz tilida). Arxivlandi asl nusxasi 2015 yil 29 yanvarda. Olingan 12 oktyabr 2015.
- ^ Breast Implant Information Booklet (PDF) (4-nashr). Kanberra: Avstraliya Hamdo'stligi. 2001 yil. ISBN 0642735794. Arxivlandi asl nusxasi (PDF) 2007-01-01 da. Olingan 2006-12-29.
- ^ "German Society for Senology, Declaration of Consensus for the Security of Silicone Breast Implants-24 September 1998". 1998 yil. Iqtibos jurnali talab qiladi
| jurnal =(Yordam bering) - ^ Janowsky EC, Kupper LL, Hulka BS (2000). "Meta-analyses of the Relation between Silicone Breast Implants and the Risk of Connective-tissue Diseases". Nyu-England tibbiyot jurnali. 342 (11): 781–790. doi:10.1056/NEJM200003163421105. PMID 10717013.
- ^ [1] Arxivlandi 2005 yil 27 dekabr, soat Orqaga qaytish mashinasi
- ^ [2] Arxivlandi 2006 yil 23 iyun, soat Orqaga qaytish mashinasi
- ^ Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B (2001). "Do silicone Breast Implants Cause Rheumatologic Disorders? A Systematic Review for a Court-appointed National Science Panel". Artrit revmi. 44 (11): 2477–84. doi:10.1002/1529-0131(200111)44:11<2477::AID-ART427>3.0.CO;2-Q. PMID 11710703.
- ^ (PDF) https://web.archive.org/web/20030829114951/http://www.eucomed.be/docs/STOA-SILICONE%20BREAST%20IMPLANT%20Study%20update-30May03.pdf. Arxivlandi asl nusxasi (PDF) 2003-08-29 kunlari. Olingan 2019-01-28. Yo'qolgan yoki bo'sh
sarlavha =(Yordam bering) - ^ Neuhann-Lorenz C, Fedeles J, Eisenman-Klein M, Kinney B, Cunningham BL (2001). "Eighth IQUAM Consensus Position Statement: Transatlantic Innovations, April 2009". Plastik va rekonstruktiv jarrohlik. 127 (3): 1368–75. doi:10.1097/PRS.0b013e318206312e. PMID 21364439. S2CID 29112694.
- ^ Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G (2000). "Prevalence of Rupture of Silicone gel Breast Implants Revealed on MR Imaging in a Population of Women in Birmingham, Alabama". Amerika Roentgenologiya jurnali. 175 (4): 1057–1064. doi:10.2214/ajr.175.4.1751057. PMID 11000165.
- ^ Walker PS, Walls B, Murphy DK (2009). "Natrelle Saline-filled Breast Implants: a Prospective 10-year Study". Estetik jarrohlik jurnali. 29 (1): 19–25. doi:10.1016/j.asj.2008.10.001. PMID 19233001.
- ^ Hölmich LR, Vejborg IM, Conrad C, Sletting S, Høier-Madsen M, Fryzek JP, McLaughlin JK, Kjøller K, Wiik A, Friis S (2004). "Untreated Silicone Breast Implant Rupture". Plastik va rekonstruktiv jarrohlik. 114 (1): 204–214. doi:10.1097/01.PRS.0000128821.87939.B5. PMID 15220594. S2CID 25947224.
- ^ Katzin WE, Centeno JA, Feng LJ, Kiley M, Mullick FG (2001). "Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation". Amerika jarrohlik patologiyasi jurnali. 29 (4): 506–11. doi:10.1097/01.pas.0000155145.60670.e4. PMID 15767806. S2CID 31982669. Arxivlandi asl nusxasi (—Olimlarni izlash) 2009 yil 24 mayda.
- ^ "Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component)". FDA Breast Implant Consumer Handbook - 2004. 2000-05-22. Arxivlandi from the original on 2007-06-09. Olingan 2007-05-04.
- ^ a b v "Local Complications". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Arxivlandi asl nusxasi 2007-05-13 kunlari. Olingan 2007-05-04.
- ^ MRI of a ruptured silicone breast implant Arxivlandi 2013-09-26 da Orqaga qaytish mashinasi 2013-04-05
- ^ Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH (2003). "Incidence of Silicone Breast Implant Rupture". Arch. Surg. 138 (7): 801–806. doi:10.1001/archsurg.138.7.801. PMID 12860765.
- ^ Hedén P, Nava MB, van Tetering JP, Magalon G, Fourie le R, Brenner RJ, Lindsey LE, Murphy DK, Walker PS (2006). "Prevalence of Rupture in Inamed Silicone Breast Implants". Plastik va rekonstruktiv jarrohlik. 118 (2): 303–308. doi:10.1097/01.prs.0000233471.58039.30. PMID 16874191. S2CID 30442865.
- ^ "FDA summary of clinical issues (MS Word document)". Arxivlandi from the original on 2008-03-08.
- ^ Cunningham B, McCue J (2009). "Safety and effectiveness of Mentor's MemoryGel implants at 6 years". Plastik va rekonstruktiv jarrohlik. 33 (3): 440–444. doi:10.1007/s00266-009-9364-6. PMID 19437068. S2CID 25722841.
- ^ Hedén P, Boné B, Murphy DK, Slicton A, Walker PS (2006). "Style 410 Cohesive Silicone Breast Implants: Safety and Effectiveness at 5 to 9 years after Implantation". Plastik va rekonstruktiv jarrohlik. 118 (6): 1281–1287. doi:10.1097/01.prs.0000239457.17721.5d. PMID 17051096. S2CID 34380204.
- ^ Hölmich LR, Fryzek JP, Kjøller K, Breiting VB, Jørgensen A, Krag C, McLaughlin JK (2005). "The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging". Plastik jarrohlik yilnomalari. 54 (6): 583–589. doi:10.1097/01.sap.0000164470.76432.4f. PMID 15900139. S2CID 39525474.
- ^ "Expert Advisory Panel on Breast Implants: Record of Proceedings". HealthCanada. 2005-09-29. Arxivlandi asl nusxasi 2007-11-07 kunlari. Olingan 2007-05-04.
- ^ Song JW, Kim HM, Bellfi LT, Chung KC (2011). "The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis". Plastik va rekonstruktiv jarrohlik. 127 (3): 1029–1044. doi:10.1097/PRS.0b013e3182043630. PMC 3080104. PMID 21364405.
- ^ AFP (18 September 2011). "Breast implants safe, but not for life: US experts". Mustaqil. Arxivlandi asl nusxasidan 2016 yil 3 avgustda.
- ^ Barnsley GP, Sigurdson LJ, Barnsley SE (2006). "Textured surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: a Meta-analysis of Randomized Controlled Trials". Plastik va rekonstruktiv jarrohlik. 117 (7): 2182–2190. doi:10.1097 / 01.prs.0000218184.47372.d5. PMID 16772915. S2CID 35420582.
- ^ Wong CH, Samuel M, Tan BK, Song C (2006). "Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: a Systematic Review". Plastik va rekonstruktiv jarrohlik. 118 (5): 1224–1236. doi:10.1097 / 01.prs.0000237013.50283.d2. PMID 17016195. S2CID 29643167.
- ^ Handel N, Gutierrez J (May 2006). "Ko'pikli poliuretan bilan qoplangan ko'krak implantlarining uzoq muddatli xavfsizligi va samaradorligi". Journal of Aesthetic Surgery. 26 (3): 265–274. doi:10.1016 / j.asj.2006.04.001. PMID 19338905.
- ^ Mladick RA (1993). ""No-touch" submuscular saline breast augmentation technique". Journal of Aesthetic Surgery. 17 (3): 183–192. doi:10.1007 / BF00636260. PMID 8213311. S2CID 39767802.
- ^ Adams WP, Rios JL, Smith SJ (2006). "Enhancing Patient Outcomes in Aesthetic and Reconstructive Breast Surgery using Triple Antibiotic Breast Irrigation: Six-year Prospective Clinical Study". Plastik va rekonstruktiv jarrohlik. 117 (1): 30–6. doi:10.1097/01.prs.0000185671.51993.7e. PMID 16404244. S2CID 35238465.
- ^ Planas J, Cervelli V, Planas G (2001). "Five-year experience on ultrasonic treatment of breast contractures". Estetik plastik jarrohlik. 25 (2): 89–93. doi:10.1007 / s002660010102. PMID 11349308. S2CID 2784003.
- ^ Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R (2002). "Zafirlukast (Accolate): kapsula kontrakturasi uchun yangi davolash". Aesthetic Plast. Surg. 22 (4): 329–36. doi:10.1067 / maj.2002.126753. PMID 19331987.
- ^ Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G (2006). "The effects of zafirlukast on capsular contracture: preliminary report". Aesthetic Plast. Surg. 30 (5): 513–520. doi:10.1007 / s00266-006-0038-3. PMID 16977359. S2CID 251008.
- ^ Silver H (1982). "Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy)". Plastik va rekonstruktiv jarrohlik. 69 (5): 802–805. doi:10.1097/00006534-198205000-00013. PMID 7071225. S2CID 8451166.
- ^ Tebbetts JB (October 2006). "Out Points Criteria for Breast Implant Removal without Replacement and Criteria to Minimize Reoperations following Breast Augmentation". Plastik va rekonstruktiv jarrohlik. 114 (5): 1258–1262. doi:10.1097/01.prs.0000136802.91357.cf. PMID 15457046.
- ^ Tebbetts JB (December 2006). "Achieving a Zero Percent Reoperation Rate at 3 years in a 50-consecutive-case Augmentation Mammaplasty Premarket Approval Study". Plastik va rekonstruktiv jarrohlik. 118 (6): 1453–7. doi:10.1097/01.prs.0000239602.99867.07. PMID 17051118. S2CID 27630646.
- ^ Lipworth L, Holmich LR, McLaughlin JK (May 2011). "Silicone breast implants and connective tissue disease: no association". Immunopatologiya bo'yicha seminarlar. 33 (3): 287–94. doi:10.1007/s00281-010-0238-4. PMID 21369953. S2CID 22297654.
- ^ Arepalli SR, Bezabeh S, Brown SL (2002). "Allergic reaction to platinum in silicone breast implants". Journal of Long-term Effects of Medical Implants. 12 (4): 299–306. doi:10.1615/jlongtermeffmedimplants.v12.i4.80. PMID 12627791.
- ^ "FDA Backgrounder on Platinum in Silicone Breast Implants". AQSh oziq-ovqat va farmatsevtika idorasi. 16 iyun 2006. Arxivlangan asl nusxasi on 2006-07-15.
- ^ Komissar. "Safety Alerts for Human Medical Products - Breast Implants: Update - Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)". www.fda.gov. Arxivlandi asl nusxasidan 2018 yil 28 aprelda. Olingan 28 aprel 2018.
- ^ Sverdlov, Stiven X.; Kampo, Elias; Pileri, Stefano A.; Xarris, Nensi Li; Shteyn, Xarald; Ziber, Reyner; Advani, Ranjana; Gielmini, Mishel; Salles, Gilles A .; Zelenets, Endryu D.; Jaffe, Elaine S. (2016-05-19). "Jahon sog'liqni saqlash tashkiloti lenfoid neoplazmalar tasnifini 2016 yilda qayta ko'rib chiqish". Qon. 127 (20): 2375–2390. doi:10.1182 / qon-2016-01-643569. ISSN 0006-4971. PMC 4874220. PMID 26980727.
- ^ Sog'liqni saqlash, asboblar va radiologik markaz. "Breast Implants - Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma". www.fda.gov. Arxivlandi asl nusxasidan 2018 yil 28 aprelda. Olingan 28 aprel 2018.
- ^ Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, et al. (2014 yil yanvar). "Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients". Klinik onkologiya jurnali. 32 (2): 114–20. doi:10.1200/JCO.2013.52.7911. PMC 4062709. PMID 24323027.
- ^ a b Clemens, Mark. "Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) Arxivlandi 2017-03-26 da Orqaga qaytish mashinasi " (2017).
- ^ Clemens MW, Horwitz SM (March 2017). "NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma". Estetik jarrohlik jurnali. 37 (3): 285–289. doi:10.1093/asj/sjw259. PMID 28184418.
- ^ "Implant-associated ALCL Facts | The MD Anderson Foundation". www.mdanderson.org. Arxivlandi asl nusxasidan 2017-12-09. Olingan 2017-12-08.
- ^ "Breast Implant Associated ALCL: PROFILE Project | The Plastic Surgery Foundation". www.thepsf.org. Arxivlandi asl nusxasidan 2017-05-07. Olingan 2017-04-25.
- ^ Johnson GW, Christ JE (1993). "The Endoscopic Breast augmentation: The Transumbilical Insertion of Saline-filled Breast Implants". Plastik va rekonstruktiv jarrohlik. 92 (5): 801–8. doi:10.1097/00006534-199392050-00004. PMID 8415961.
- ^ Wallach SG (2004). "Maximizing the Use of the Abdominoplasty Incision". Plastik va rekonstruktiv jarrohlik. 113 (1): 411–417. doi:10.1097/01.PRS.0000091422.11191.1A. PMID 14707667. S2CID 44430032.
- ^ Graf RM, Bernardes A, Rippel R, Araujo LR, Damasio RC, Auersvald A (2003). "Subfascial Breast Implant: A New Procedure". Plastik va rekonstruktiv jarrohlik. 111 (2): 904–908. doi:10.1097/01.PRS.0000041601.59651.15. PMID 12560720.
- ^ Tebbetts JB (2004). "Does Fascia Provide Additional, Meaningful Coverage over a Breast Implant?". Plastik va rekonstruktiv jarrohlik. 113 (2): 777–779. doi:10.1097/01.PRS.0000104516.13465.96. PMID 14758271.
- ^ Tebbetts JB (2002). "A System for Breast Implant Selection Based on Patient Tissue Characteristics and Implant-soft tissue Dynamics". Plastik va rekonstruktiv jarrohlik. 109 (4): 1396–1409. doi:10.1097/00006534-200204010-00030. PMID 11964998.
- ^ Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty: safety and efficacy of indwelling catheters in 644 consecutive patients". Aesthet Surg J. 28 (3): 279–84. doi:10.1016/j.asj.2008.02.001. PMID 19083538.
- ^ Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: data analysis". Aesthet Surg J. 28 (6): 631–41. doi:10.1016/j.asj.2008.09.001. PMID 19083591.
- ^ Tebbetts JB (2002). "A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics". Plast. Namoyish. Surg. 109 (4): 1396–409, discussion 1410–5. doi:10.1097/00006534-200204010-00030. PMID 11964998.
- ^ Tebbetts JB, Adams WP (2005). "Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process". Plast. Namoyish. Surg. 116 (7): 2005–16. doi:10.1097/01.prs.0000191163.19379.63. PMID 16327616. S2CID 11180810.
- ^ Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: part I. Refining practices by using motion and time study principles". Plast. Namoyish. Surg. 109 (1): 273–90, discussion 291–2. doi:10.1097/00006534-200201000-00044. PMID 11786826. S2CID 26419990.
- ^ Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: Part II. Patient preparation, refined surgical techniques, and instrumentation". Plast. Namoyish. Surg. 109 (1): 293–305, discussion 306–7. doi:10.1097/00006534-200201000-00046. PMID 11786828. S2CID 21392313.
- ^ "Choosing Your Breast Implants" (Internet). Minneapolis Plastic Surgery, LTD. Arxivlandi asl nusxasidan 2016 yil 24 noyabrda. Olingan 23 noyabr 2016.
- ^ Zannis J (2017). Tales for Tagliacozzi: An Inside Look at Modern-Day Plastic Surgery. ISBN 9781524659073. Olingan 7 iyun 2019.
- ^ a b Stevens WG, Hirsch EM, Stoker DA, Cohen R (2006). "In vitro Deflation of Pre-filled Saline Breast Implants". Plastik va rekonstruktiv jarrohlik. 118 (2): 347–349. doi:10.1097/01.prs.0000227674.65284.80. PMID 16874200. S2CID 41156555.
- ^ Arion HG (1965). "Retromammary Prosthesis". C R Société Française de Gynécologie. 5.
- ^ Eisenberg TS (2009). "Silicone Gel Implants Are Back — So What?". Amerika kosmetik jarrohlik jurnali. 26: 5–7. doi:10.1177/074880680902600103. S2CID 136191732.
- ^ Kronin TD, Gerov FJ (1963). "Mammaplastika kattalashtirish:" Protez "ning yangi" tabiiy tuyg'usi ". Excerpta Medica xalqaro kongresslar seriyasi. 66: 41.
- ^ Luu XM, Xutter JK, Bushar HF (1998). "Ko'pikli poliuretan bilan qoplangan ko'krak implantlaridan yuvilgan 2,4-toluendiamin uchun fiziologik asoslangan farmakokinetik model". Atrof-muhit salomatligi istiqboli. 106 (7): 393–400. doi:10.2307/3434066. JSTOR 3434066. PMC 1533137. PMID 9637796.
- ^ Xester TR, Tebbetts JB, Maksvell GP (2001). "Poliuretan bilan qoplangan sut bezlari protezi: faktlar va fantastika (II): orqaga qarash va oldinga" ko'z ". Klinik plastik jarrohlik. 28 (3): 579–86. doi:10.1016 / S0094-1298 (20) 32397-X. PMID 11471963.
- ^ Jigarrang MH, Shenker R, Silver SA (2005). "Ko'krak estetik va rekonstruktiv jarrohlik jarayonida birlashtiruvchi silikon jel ko'krak implantlari". Plastik va rekonstruktiv jarrohlik. 116 (3): 768-779, munozara 779-1. doi:10.1097 / 01.prs.0000176259.66948.e7. PMID 16141814. S2CID 35392851.
- ^ Fruhstorfer BH, Xojson EL, Malata CM (2004). "Ko'krak bezi implantatsiyasining estetik va rekonstruktiv operatsiyasida anatomik yumshoq birlashuvchi silikon jel protezi bilan dastlabki tajriba". Plastik jarrohlik yilnomalari. 53 (6): 536–542. doi:10.1097 / 01.sap.0000134508.43550.6f. PMID 15602249. S2CID 24661896.
- ^ Xeden P, Jernbek J, Xober M (2001). "Anatomik yopishqoq jel implantlari bilan ko'krakni kattalashtirish: dunyodagi eng katta hozirgi tajriba". Plastik jarrohlik klinikalari. 28 (3): 531–552. doi:10.1016 / S0094-1298 (20) 32393-2. PMID 11471959.
- ^ a b v Nichter LS, Hardesty RA, Anigian GM (iyul 2018). "IDEAL IMPLANT Tuzilgan ko'krak implantlari: 6 yoshdagi asosiy o'rganish natijalari". Plastik va rekonstruktiv jarrohlik. 142 (1): 66–75. doi:10.1097 / PRS.0000000000004460. PMC 6045953. PMID 29489559.
- ^ "Ko'krak implantlarining qaysi turlari mavjud?". Amerika plastik jarrohlar jamiyati.
- ^ Ko'krak jarrohligidan keyin emizish Arxivlandi 2010-12-30 da Orqaga qaytish mashinasi, La Leche League, ma'lumotnomalarni o'z ichiga oladi.
- ^ Emizish va ko'krak implantlari Arxivlandi 2010-12-31 da Orqaga qaytish mashinasi, Tanlangan bibliografiya 2003 yil aprel, LLLI emizish bo'yicha ma'lumot markazi
- ^ a b Anorganik sut: Kendra Uilkinson implantlari bo'lsa ham, bolasini emizishi mumkinmi? Arxivlandi 2010-01-25 da Orqaga qaytish mashinasi, Kristofer Beam, Slate.com, 2009 yil 11-dekabr
- ^ Handel N, Silverstayn MJ, Gamagami P, Jensen JA, Kollinz A (1992). "Mammaplastikani kattalashtirishdan keyin ko'krakning mamografik vizualizatsiyasiga ta'sir qiluvchi omillar". JAMA. 268 (14): 1913–1917. doi:10.1001 / jama.268.14.1913. PMID 1404718.
- ^ O'Keefe JR, Wilkinson JM, Spuur KM (iyun 2020). "Avstraliyada kattalashgan ko'krakni mamografik tasvirlash bo'yicha amaldagi amaliyot". Tibbiy radiatsiya fanlari jurnali. 67 (2): 102–110. doi:10.1002 / jmrs.374. PMC 7276184. PMID 31981297.
- ^ Klark CP, Peters GN, O'Brien KM (1993). "Kattalashgan ko'krakdagi saraton: diagnostika va prognoz". Saraton. 72 (7): 2170–4. doi:10.1002 / 1097-0142 (19931001) 72: 7 <2170 :: AID-CNCR2820720717> 3.0.CO; 2-1. PMID 8374874.
- ^ Skinner KA, Silberman H, Dougherty V, Gamagami P, Vaysman J, Sposto R, Silverstayn MJ (2001). "Kattalashtirilgan mammoplastikadan so'ng ko'krak bezi saratoni". Ann Surg Oncol. 8 (2): 138–44. doi:10.1007 / s10434-001-0138-x. PMID 11258778. S2CID 26010159.
- ^ a b Le GM, O'Malley CD, Glaser SL, Lynch CF, Stenford JL, Keegan TH, West DW (2005). "Ko'krak bezi saratonining dastlabki bosqichida bo'lgan ayollarda mastektomiyadan so'ng ko'krak implantlari: tarqalishi va yashashga ta'siri". Ko'krak bezi saratoni rez. 7 (2): R184-93. doi:10.1186 / bcr974. PMC 1064128. PMID 15743498.
- ^ Handel N, Silverstayn MJ (2006). "Kattalashtirilgan ayollarda ko'krak bezi saratoni diagnostikasi va prognozi". Plastik va rekonstruktiv jarrohlik. 118 (3): 587–93. doi:10.1097 / 01.prs.0000233038.47009.04. PMID 16932162. S2CID 36277679.
- ^ Kanningem B (2006). "Kattalashtirilgan ayollarda ko'krak bezi saratoni diagnostikasi va prognozi - Muhokama". Plastik va rekonstruktiv jarrohlik. 118 (3): 594–595. doi:10.1097 / 01.prs.0000233047.87102.8e.
- ^ Shvarts GF, Veronesi U, Kloub KB, Dikson JM, Fentiman IS, Heyvang-Köbrunner SH, Holland R, Xyuz KS, Mansel RE, Margolese R, Mendelson EB, Olivotto IA, Palazzo JP, Solin LJ (2006). "Ko'krakni saqlash bo'yicha konsensus konferentsiyasi". JAKAS. 203 (2): 198–207. doi:10.1016 / j.jamcollsurg.2006.04.009. PMID 16864033.
- ^ Lavigne E, Holowaty EJ, Pan SY, Villeneuve PJ, Johnson KC, Fergusson DA va boshq. (2013 yil aprel). "Ko'krak bezi implantlari qo'yilgan ayollar orasida ko'krak bezi saratonini aniqlash va omon qolish: kuzatuv tadqiqotlarini tizimli ko'rib chiqish va meta-tahlil". BMJ. 346 (aprel 1): f2399. doi:10.1136 / bmj.f2399. PMID 23637132.
- ^ Xvan ES, Lichtensztajn DY, Gomes SL, Fowble B, Clarke CA (aprel 2013). "Ko'krak bezi saratonining dastlabki bosqichida lumpektomiya va mastektomiyadan so'ng omon qolish: yosh va gormon retseptorlari holatining ta'siri". Saraton. 119 (7): 1402–11. doi:10.1002 / cncr.27795. PMC 3604076. PMID 23359049.
- ^ Tserniy V (1895). "Plastischer Ersatz der Brusthus durch ein Lipoma". Zentralblatt für Chirurgie. 27: 72.
- ^ Glicenshteyn J (2007 yil aprel). "[Birinchi" plomba ", vazelin va kerosin. Mo''jizadan falokatga qadar]". Annales de Chirurgie Plastique et Esthétique. 52 (2): 157–61. doi:10.1016 / j.anplas.2006.05.003. PMID 16860452.
- ^ Tibbiyot instituti (AQSh) Silikon ko'krak implantlari xavfsizligi qo'mitasi, Bondurant S, Ernster V, Herdman R va boshq. (Silikon ko'krak implantatsiyasining xavfsizligi qo'mitasi) (1999). Bondurant S, Ernster V, Xerdman R (tahrir). Silikon ko'krak implantatsiyasining xavfsizligi. Tibbiyot instituti. p. 21. doi:10.17226/9602. ISBN 0-309-06532-1. PMID 20669503. Arxivlandi asl nusxasidan 2007-03-13.
- ^ Anderson N (1997). "Sud ishi bo'yicha fan: Ko'krak silikonining joylashtirilishi bo'yicha tortishuvdan saboqlar". Nyu-York yuridik fakultetining yuridik sharhi. 41 (2): 401–07.
- ^ a b v d e f g h FDA Ko'krak implantatsiyasini iste'molchilar uchun qo'llanma - 2004 yil Arxivlandi 2008-09-17 da Orqaga qaytish mashinasi
- ^ Tibbiyot instituti (AQSh) Silikon ko'krak implantlari xavfsizligi qo'mitasi, Bondurant S, Ernster V, Herdman R (1999). Silikon ko'krak implantlarining xavfsizligi - Milliy akademiyalar matbuot. nap.edu. doi:10.17226/9602. ISBN 978-0-309-15740-7. PMID 20669503.
- ^ Grigg M, Bondurant S, Ernster VL, Herdman R (2000). Grigg M, Bondurant S, Ernster VL, Herdman R (tahrir). Silikon ko'krak implantatsiyasining xavfsizligi to'g'risida ayollar uchun ma'lumot - Milliy akademiyalar matbuot. nap.edu. doi:10.17226/9618. ISBN 978-0-309-06593-1. PMID 20669498.
- ^ FDA o'rganish Arxivlandi 2008 yil 13 yanvar, soat Orqaga qaytish mashinasi
- ^ "FDA tomonidan tasdiqlash". fda.gov. Arxivlandi asl nusxasidan 2009 yil 30 martda. Olingan 28 aprel 2018.
- ^ "FDA tomonidan tasdiqlash". fda.gov. Arxivlandi asl nusxasidan 2009 yil 26 mayda. Olingan 28 aprel 2018.
- ^ "FDA ko'krakda silikon jel bilan to'ldirilgan implantlarni ma'qullaydi". FDA. Arxivlandi asl nusxasidan 2008-07-26. Olingan 2008-07-01.
- ^ a b "Ko'krak bezi implantatsiyasiga oid savollar va javoblar". AQSh oziq-ovqat va farmatsevtika idorasi. Oktyabr 2010. Arxivlangan asl nusxasi 2010-11-12 kunlari.