Shpindelni tekshirish punkti - Spindle checkpoint

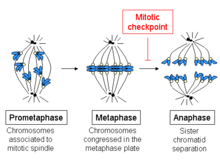
The milni tekshirish punkti, deb ham tanilgan metafazadan anafazaga o'tish, milni yig'ish nazorat punkti (SAC)yoki mitotik nazorat punkti, a hujayra siklini tekshirish punkti davomida mitoz yoki mayoz bu takrorlanadigan nusxaning ajratilishini oldini oladi xromosomalar (anafaza ) har bir xromosoma to'g'ri biriktirilguncha mil. To'g'ri ajratishga erishish uchun ikkalasi kinetoxoralar opa-singilga xromatidlar qarama-qarshi milya ustunlariga biriktirilgan bo'lishi kerak (bipolyar yo'nalish).[1] Faqatgina ushbu biriktirma namunasi har bir qizi bo'lishini ta'minlaydi hujayra xromosomaning bitta nusxasini oladi. Ushbu nazorat punktining aniqlovchi biokimyoviy xususiyati - bu stimulyatsiya anafazani rivojlantiruvchi kompleks tomonidan M-faza siklin-CDK komplekslari, bu o'z navbatida sabab bo'ladi proteolitik yo'q qilish tsiklinlar va ushlab turadigan oqsillar opa-singil xromatidlar birgalikda.[2]
Umumiy nuqtai va ahamiyati
Metafazaning boshlanishi mikrotubulalarning xromosomalarning kinetoxoralariga ulanishi, shuningdek xromosomalarning hujayraning o'rtasida joylashganligi bilan tavsiflanadi. Har bir xromatidaning o'ziga xos kinetoxori bor va opa-singil xromatidalarning kinetoxorlari bilan bog'langan barcha mikrotubulalar hujayraning qarama-qarshi qutblaridan nurlanadi. Ushbu mikrotubulalar xromosomalarga hujayralarning qarama-qarshi uchlariga tortish kuchini o'tkazadi, singil xromatidlar orasidagi uyg'unlik esa bu kuchga qarshi turadi.
Metafazadan anafaza o'tishda singil xromatidlar orasidagi bu birlashma eriydi va ajratilgan xromatidlar shpindel mikrotubulalari tomonidan hujayraning qarama-qarshi tomonlariga tortiladi. Xromatidlar yana shpindel qutblarining jismoniy harakati bilan ajralib turadi. Xromatidlarning barvaqt dissotsiatsiyasi qiz hujayralarida xromosomalarning noto'g'ri ajratilishiga va aneuploidiyaga olib kelishi mumkin. Shunday qilib, metafazani nazorat qilish punktining vazifasi xromosomalar to'g'ri biriktirilgunga qadar, opa-singil xromatidlar ajratilguncha anaafazaga o'tishni oldini olishdir.
Hujayraning o'ziga xosligini va to'g'ri ishlashini saqlab qolish uchun tegishli sonini saqlash kerak xromosomalar har biridan keyin hujayraning bo'linishi. Kutilganidan kam yoki ko'p miqdordagi xromosomalarga ega bo'lgan qiz hujayralarini yaratishda xato (vaziyat shunday nomlangan) aneuploidiya ), eng yaxshi holatda hujayraning o'limiga olib kelishi yoki muqobil ravishda bu halokatli bo'lishi mumkin fenotipik natijalar.[3][4] Bunga misollar:
- Saraton hujayralarida, aneuploidiya tez-tez sodir bo'ladigan hodisa bo'lib, bu hujayralar ishtirok etgan texnikada nuqson borligini ko'rsatadi xromosomalarning ajratilishi, shuningdek, ajratishning to'g'ri bajarilishini ta'minlaydigan mexanizmda.
- Odamlarda, Daun sindromi hujayralarida bitta qo'shimcha nusxasini olib yuradigan bolalarda paydo bo'ladi 21-xromosoma, nuqson natijasida xromosomalarning ajratilishi davomida mayoz ajdodlardan birida. Ushbu nuqson a hosil qiladi jinsiy hujayralar (spermatozoid yoki oosit) qo'shimcha xromosoma bilan 21. Keyin urug'lantirish, bu jinsiy hujayralar an hosil qiladi embrion xromosoma 21 ning uchta nusxasi bilan.
Shpindelni yig'ish punktining kashf etilishi (SAC)

Zirkl (1970 yilda) birinchilardan bo'lib metafaza plastinkasiga etib kelish uchun bitta xromosoma sustlashganda, anafaza boshlanishi u kelganidan bir necha daqiqagacha qoldirilishini kuzatgan.[5] Ushbu kuzatish shunga o'xshashlar bilan birgalikda metafazadan anafazaga o'tishda boshqarish mexanizmi mavjudligini ko'rsatdi. Kabi giyohvand moddalarni iste'mol qilish nokodazol va kolxitsin, mitotik shpindel demontaj qilinadi va hujayra tsikli metafazadan anafazaga o'tishda bloklanadi. Ushbu dorilarni qo'llash (1992 yilda Rieder va Palazzo-ning sharhiga qarang[6]), taxminiy boshqarish mexanizmi nomlandi Shpindelni yig'ish punkti (SAC). Ushbu tartibga solish mexanizmi shundan beri intensiv ravishda o'rganilmoqda.[7]
Turli xil genetik tadqiqotlar yordamida turli xil nuqsonlar SACni faollashtirishi mumkinligi aniqlandi: shpindel depolimerizatsiyasi,[8][9] dicentrik xromosomalarning mavjudligi (ikki sentromer bilan),[10] beparvolik bilan ajratib turadigan sentromeralar,[11] mil qutblari tanasidagi nuqsonlar S. cerevisiae,[12] kinetoxor oqsillaridagi nuqsonlar,[13] tsentromerik DNKdagi mutatsiyalar[14] yoki nuqsonlari molekulyar motorlar mitoz paytida faol.[8] Ushbu kuzatishlarning qisqacha mazmuni 1999 yilda Xardvik va uning hamkorlari maqolasida keltirilgan.[15]
O'zining kuzatuvlaridan foydalanib, Zirkl[5] birinchi bo'lib "hujayraning anafazaga o'tishi uchun zarur bo'lgan ba'zi bir (...) moddalar C dan bir necha daqiqa o'tgach (oxirgi xromosomaning metafaza plitasiga tushish paytidan) keyin paydo bo'lishi yoki sitoplazmatik , bu funktsiya mitoz shpindelga biriktirilmagan kinetoxorlarda joylashganligini ko'rsatuvchi C "da yoki C dan keyin darhol", McIntosh ushbu taklifni ilgari surdi, ya'ni sentromeralarda joylashgan kuchlanishga sezgir bo'lgan bitta ferment anafaza boshlanishiga inhibitor ishlab chiqaradi. ikkita singil kinetoxora bipolyar kuchlanish ostida emas.[16] Darhaqiqat, mavjud ma'lumotlar "anafazaga kirishni kuting" signali asosan biriktirilmagan kinetoxorlarda yoki unga yaqin joyda ishlab chiqarilishini taxmin qilmoqda.[17] Biroq, milga kinetoxor biriktirilishi bilan bog'liq bo'lgan, inhibitoryal signalni inaktivatsiyalashga va metafazni to'xtatib turishga qodir bo'lgan birlamchi hodisa kinetoxor tomonidan mikrotubulalarni sotib olish bo'lishi mumkin (Rieder va sheriklar tomonidan 1995 yilda taklif qilinganidek)[17]) yoki mikrotubulalarning kinetoxorlarga o'rnatilishini barqarorlashtiruvchi kuchlanish (Niklas laboratoriyasida amalga oshirilgan tajribalar taklifiga binoan).[18]). Tabanda ikkita mustaqil mitotik shpindel bo'lgan hujayralardagi keyingi tadqiqotlar sitoplazma metafazadan anafaza o'tishining inhibitori biriktirilmagan kinetoxorlar tomonidan hosil bo'lishini va sitoplazmada erkin tarqalmasligini ko'rsatdi.[19] Shunga qaramay, xuddi shu tadqiqotda hujayraning bir qismida metafazadan anafazaga o'tish boshlangandan so'ng, bu ma'lumotlar butun sitoplazma va biriktirilmagan kinetoxorlarni o'z ichiga olgan ikkinchi shpindel bilan bog'liq bo'lgan "anafazaga kirishni kuting" signalini engib chiqishi mumkin.
Opa-singil xromatidlarni ko'paytirish, birlashish va ajratish to'g'risida ma'lumot
Hujayraning bo'linishi: materialning takrorlanishi va qiz hujayralariga tarqalishi

Hujayralar bo'linishga tayyor bo'lganda, chunki hujayra kattaligi etarlicha katta yoki ular tegishli stimulni oladilar,[20] ular hujayra tsikliga kirish mexanizmini faollashtiradi va S (sintez) bosqichida ko'pchilik organoidlarni ko'paytiradi, shu jumladan ularning tsentrosoma. Shuning uchun, hujayraning bo'linish jarayoni tugagach, har bir qiz hujayra organellalarning to'liq to'plamini oladi. Shu bilan birga, S fazasi davomida barcha hujayralar o'zlarining nusxalarini ko'paytirishlari kerak DNK juda aniq, jarayon deb nomlangan DNKning replikatsiyasi. DNKning replikatsiyasi tugagandan so'ng, eukaryotlarda DNK molekulasi zichlanib, kondensatsiyalanib mitoz hosil qiladi xromosomalar, ularning har biri ikkita singil tomonidan tashkil etilgan xromatidlar, tashkil etish bilan birga ushlab turiladigan hamjihatlik ular orasida; har bir xromatid to'liq DNK molekulasidir mikrotubulalar hujayraning qarama-qarshi qutblarida joylashgan bo'linadigan hujayraning ikki sentrosomasidan biriga. Sentrosomalar va mikrotubulalar hosil qilgan tuzilishga nom berilgan mitotik mil, xarakterli shakli tufayli, xromosomalarni ikki sentrosoma o'rtasida ushlab turadi. Ikkala opa-singil kromatidlar ham shu vaqtgacha birga bo'lishadi anafaza; hozirgi paytda ular bir-biridan ajralib, ular biriktirilgan santrosoma tomon sayohat qilishadi. Shu tarzda, bo'linish jarayoni oxirida ikkita qiz hujayralar ajralib chiqqanda, ularning har biri to'liq xromatidlar to'plamini oladi. Hujayraning bo'linishi paytida opa-singil xromatidlarning to'g'ri tarqalishi uchun javobgar bo'lgan mexanizm nomlangan xromosomalarning ajratilishi.
Xromosomalarning ajralishi to'g'ri bo'lishini ta'minlash uchun hujayralar aniq va murakkab mexanizmni ishlab chiqdilar. Birinchi navbatda hujayralar muvofiqlashtirishi kerak tsentrosoma DNK replikatsiyasi bilan takrorlanish va bu koordinatsiyadagi muvaffaqiyatsizlik monopolyar yoki ko'p qutbli mitotik shpindellarni hosil qiladi, bu odatda g'ayritabiiy xromosomalarni ajratishini keltirib chiqaradi,[21] chunki bu holda xromosomalarning tarqalishi muvozanatli tarzda bo'lmaydi.
Mitoz: xromosomalarni milga mahkamlash va xromosomalarning ajratilishi

S fazasi davomida tsentrosoma takrorlashni boshlaydi. Faqat mitozning boshida, ikkalasi ham sentriol ularning maksimal uzunligiga erishish, qo'shimcha materiallar jalb qilish va mikrotubulalarni nukleatsiya qilish qobiliyati oshadi. Mitoz rivojlanishi bilan ikkala sentrosoma ham ajralib mitoz shpindelni hosil qiladi.[22] Shu tarzda, mitotik shpindel mikrotubulalarni chiqaradigan ikkita qutbga ega. Mikrotubulalar (MT) uzun protein tolalari bo'lib, ularning uchlari assimetrikdir: bir uchi "minus" (-) uchi deb nomlanadi, nisbatan barqaror va sentrosomaga yaqin, uchi esa "ortiqcha" (+) uchi deb nomlanadi, o'sish bosqichlari o'zgaruvchan va orqaga tortish, xromosomalarni qidiradigan hujayraning markazini o'rganish. Har biri xromatid deb nomlangan maxsus mintaqaga ega tsentromer, uning ustiga proteik struktura yig'ilib yig'ilgan kinetoxora, bu mikrotubulani ortiqcha uchini barqarorlashtirishga qodir. Shuning uchun, agar tasodifan hujayraning markazini o'rganayotgan mikrotubula kinetoxorga duch kelsa, kinetoxora uni egallab olishi mumkin, shunda xromosoma singlisi xromatidlaridan birining kinetoxori orqali milga bog'lanib qoladi. Kinetoxorlarni shpindelga yopishtirishda xromosoma faol rol o'ynaydi. Xromatin bilan bog'langan - Ran guaninli nukleotid almashinuvi faktori (GEF), bu xromosoma yaqinidagi sitosolik Ranni YaIM o'rniga GTP ni bog'lash uchun rag'batlantiradi. Ranning faollashtirilgan GTP bilan bog'langan shakli sitozoldagi protein komplekslaridan TPX2 kabi mikrotubulalarni stabillashadigan oqsillarni chiqaradi, bu xromosomalar atrofida mikrotubulalarning nukleatsiyasi va polimerizatsiyasini keltirib chiqaradi.[23] Ushbu kinetoxordan olingan mikrotubulalar, tashqi kinetoxordagi kinesin motorli oqsillari bilan birga, shpindel qutbidan olingan mikrotubulaning lateral yuzasi bilan o'zaro ta'sirni osonlashtiradi. Ushbu lateral qo'shimchalar beqaror, ammo ularni so'nggi qo'shimchaga aylantirish kerak. Yon tomondan oxirigacha biriktirilish mikrotubulaning plyus-uchlari o'sishi va qisqarishini to'g'ri ikki yo'nalishga erishish uchun xromosomalarni itaruvchi va tortib oluvchi kuchlarga aylantirishga imkon beradi. Shunday bo'ladiki, singil xromatidlar bir-biriga bog'langan va ikkala kinetoxor ikkala xromatidada orqada joylashgan bo'lib, bitta kinetoxor bitta sentrosomaga yopishganida, singil kinetoxor qarama-qarshi qutbda joylashgan sentrosomaga duchor bo'ladi; shu sababli, aksariyat hollarda ikkinchi kinetoxora mikrotubulalari orqali qarama-qarshi qutbdagi sentrosoma bilan bog'lanib qoladi,[24] shuning uchun xromosomalar "ikki yo'naltirilgan" bo'lib, asosiy konfiguratsiya (shuningdek, nomlangan) amfitelik) hujayra bo'linib ketganda xromosomalarning ajratilishi to'g'ri bo'lishini ta'minlash.[25][26] Ba'zan, ikkita opa-singil kinetoxordan biri bir vaqtning o'zida ikkala qutb tomonidan ishlab chiqarilgan MT-larga qo'shilishi mumkin, bu konfiguratsiya merotelic, bu milni nazorat qilish punkti tomonidan aniqlanmagan, ammo anafaza paytida orqada qolgan xromosomalarni va shuning uchun aneuploidiyani keltirib chiqarishi mumkin. Merotel yo'nalishi (opa-singil kinetoxoralar o'rtasida keskinlikning yo'qligi bilan tavsiflanadi) mitozning boshlanishida tez-tez uchraydi, ammo Aurora B oqsil (xamirturushdan umurtqali hayvonlargacha saqlanib qolgan kinaza) bu turdagi ankrajni aniqlaydi va yo'q qiladi.[27] (Izoh: Aurora B turli xil o'smalarda tez-tez haddan tashqari ta'sirlanib turadi va hozirda saratonga qarshi dorilarni ishlab chiqish maqsadidir.[28])
Mitoz paytida opa-singil xromatid birikmasi
Kohesin: SMC oqsillari
Yuqorida ta'kidlab o'tilganidek, opa-singil xromatidlar S fazadan (DNK replikatsiya qilinib, ikkita bir xil nusxada, ikkita xromatidani hosil qilishda) anafazgacha bog'lanib turadilar. Bu vaqtda ikkala opa-singil xromatidalar ajralib, bo'linayotgan hujayradagi qarama-qarshi qutblarga boradi. Xamirturush va tuxum ekstraktlaridagi genetik va biokimyoviy tadqiqotlar Ksenopus laevis birodar xromatidlarning birlashuvida muhim rol o'ynaydigan poliprotein kompleksini aniqladi (2000 yilda Xiranoning sharhiga qarang.[29]). Ushbu majmua kohesin murakkab va Saccharomyces cerevisiae kamida to'rtta kichik birlikdan iborat: Smc1p, Smc3p, Scc1p (yoki Mcd1p) va Scc3p. Smc1p ham, Smc3p ham oqsillar oilasiga mansub Xromosomalarning tarkibiy tuzilishi (SMC), ular xromosomalar guruhini tashkil qiladi ATPazalar yuqori darajada saqlanib, heterodimer hosil qiladi (Smc1p / Smc3p). Scc1p - bu homolog S.cerevisiae birinchi bo'lib ishtirok etgan oqsil sifatida aniqlangan Rad21 ning DNKni tiklash yilda S. pombe. Ushbu to'rtta oqsil xamirturushda juda muhimdir va ularning har qandayida mutatsiya singil xromatididan ajralishni keltirib chiqaradi. Xamirturushda kohesin xromosoma qo'llari bo'ylab imtiyozli joylar bilan bog'lanadi va sentromeralarga juda yaqin, bu xromatin immunoprecipitatsiyasi yordamida o'tkazilgan tadqiqotda ko'rsatilgandek.[30]
Heteroxromatinning roli
Klassik sitologik kuzatishlar shuni ko'rsatadiki, opa-singil xromatidlar bir-biriga ko'proq bog'langan heteroxromatik mintaqalar,[31] va bu heteroxromatinning maxsus tuzilishi yoki tarkibi kohesinni yollashni afzal ko'rishi mumkinligini ko'rsatdi.[32] Aslida Swi6 (HP-1 gomologi in S. pombe) metilat bilan bog'lanadi Lys 9 ning histon H3 va kohesinning tsentromerik takrorlanish bilan bog'lanishiga yordam beradi S. pombe.[33][34] Yaqinda o'tkazilgan tadqiqotlar shuni ko'rsatadiki RNAi mashinasozlik heteroxromatin ishlab chiqarishni tartibga soladi, bu esa o'z navbatida koezinni ushbu mintaqaga jalb qiladi S. pombe[35] va umurtqali hujayralarda.[36] Ammo sentromeralarda kuchaygan birlashishni ta'minlash uchun heteroxromatindan boshqa mexanizmlar bo'lishi kerak, chunki S. cerevisiae sentromeralar yonida heteroxromatin yo'q, ammo funktsional sentromeraning mavjudligi qo'shni mintaqada koeffin assotsiatsiyasining ko'payishiga olib keladi va 20-50 kb ni tashkil qiladi.[37]
Ushbu yo'nalishda, Orc2 (tarkibiga bitta protein kiradi kelib chiqishni aniqlash kompleksi, ORC, boshlashga aloqador DNKning replikatsiyasi davomida S bosqichi ) shuningdek, inson hujayralarida mitoz paytida kinetoxorlarda joylashgan;[38] ushbu lokalizatsiya bilan kelishilgan holda, ba'zi kuzatishlar xamirturushdagi Orc2 singil xromatid birlashmasiga bog'liqligini ko'rsatadi va uning olib tashlanishi SAC faolligini keltirib chiqaradi.[39] Shuningdek, ORC kompleksining boshqa tarkibiy qismlari (masalan, orc5 in.) Kuzatilgan S. pombe) birlashishga bog'liq.[40] Biroq, ORC oqsillarini o'z ichiga olgan molekulyar yo'l kohesinlar yo'liga qo'shimcha bo'lib ko'rinadi va bu asosan noma'lum.
Birlashish funktsiyasi va uni eritish

Sentromerik birlashish milya mikrotubulalari tomonidan qutblar tomon yo'naltiradigan kuchlarga qarshilik ko'rsatadi, bu esa opa-singil kinetoxorlar o'rtasida kuchlanish hosil qiladi. O'z navbatida, bu taranglik mikrotubula-kinetoxor birikmasini stabillashtiradi, bu oqsilni ta'sir qiladigan mexanizm orqali Avrora B (ushbu masala bo'yicha sharh: Hauf va Watanabe 2004[41]).
Darhaqiqat, kohesinning hujayra darajasining pasayishi singan xromatidlarning erta ajralishini, shuningdek metafaz plastinkasida xromosoma kongressidagi nuqsonlarni va oqsillarni delokalizatsiyasini hosil qiladi. xromosoma yo'lovchi kompleksitarkibiga Aurora B oqsili kiradi.[42][43]Kohesin kompleksi uchun taklif qilingan tuzilma shuni ko'rsatadiki, bu kompleks ikkala opa-singil xromatidlarni bir-biriga bog'lab turadi.[44] Ushbu taklif qilingan strukturada kogesinning SMC tarkibiy qismlari tarkibiy rol o'ynaydi, shuning uchun SMC heterodimeri DNKni bog'laydigan oqsil sifatida ishlashi mumkin, uning konformatsiyasi tartibga solinadi. ATP.[45] Biroq, Scc1p va Scc3p tartibga soluvchi rol o'ynaydi.[29]
Yilda S. cerevisiae, Pds1p (shuningdek ma'lum sekurin ) xromatidlarning birlashishini tartibga soladi, chunki u proteazni bog'laydi va inhibe qiladi Esp1p (sepin yoki ajratish). Anafaza boshlanganda, anafazani rivojlantiruvchi kompleks (APC / C yoki siklosoma) sekurinni parchalaydi. APC / C - bu halqali E3 ubikuitin ligaz, u ubikuitin bilan yuklangan E2 ubikuitin-konjugatsiya qiluvchi fermentni jalb qiladi. Securin faqat aktivator subbirligi Cdc20 APC / C yadrosiga bog'langan bo'lsa tan olinadi. Sekurin, Cdc20 va E2 ning barchasi APC / C E2 bilan bog'langan bo'lsa, sekurinni tanlab oladi va tanlab tanazzulga uchraydi. Sekurinning parchalanishi proteaz Esp1p / separazni ajratadi, bu esa ikkita singil xromatidlarni bir-biriga bog'laydigan kohesin halqalarini parchalaydi, shuning uchun singil xromatidlarning ajralishini rag'batlantiradi.[46] Bundan tashqari, Polo / Cdc5 ekanligi ko'rsatilgan kinaz fosforilatlar serin Scc1 uchun kesish joyi yonidagi qoldiqlar va bu fosforillanish kesish faoliyatini osonlashtiradi.[47]
Ushbu texnika evolyutsiya orqali saqlanib qolgan bo'lsa-da,[48][49] umurtqali hayvonlarda kohesin molekulalarining ko'pi Polo-shunga o'xshash 1 ga bog'liq bo'lgan jarayonda APC / C mavjudligidan qat'i nazar, profazada ajralib chiqadi (PLK1 ) va Aurora B.[50] Shunga qaramay, oz miqdordagi Scc1 metafazaga qadar inson hujayralaridagi sentromeralar bilan bog'liq bo'lib qoladi va shunga o'xshash miqdor sentromeralardan yo'qolganda anafazada kesiladi.[51] Boshqa tomondan, ba'zi bir tajribalar shuni ko'rsatadiki, singil xromatidlarning qo'llaridagi uyg'unligi singil tsentromeralar ajratilgandan so'ng asta-sekin yo'qoladi va opa-singil xromatidlar hujayraning qarama-qarshi qutblariga qarab harakatlanadi.[52][53]
Ba'zi kuzatuvlarga ko'ra, xromosoma qo'llaridagi koezinlarning bir qismi va sentromerik koezinlar oqsil bilan himoyalangan Shugoshin (Sgo1), ularning profaza paytida chiqarilishidan saqlanish.[54][55] Centromericheskiy birlashma himoyachisi sifatida ishlash uchun Sgo1 ni anafaza boshida, shuningdek Pds1p inaktiv qilish kerak. Aslida, Pds1p ham, Sgo1 ham umurtqali hayvonlardagi APC / C substratidir.[56]
Shpindelni yig'ish punktiga umumiy nuqtai
Shpindelni yig'ish punkti (SAC) noto'g'ri biriktirilgan holda ishlab chiqarilgan faol signaldir kinetoxoralar, bu hamma saqlanib qolgan eukaryotlar. SAC CDC20 ni salbiy regulyatsiya qilish orqali hujayra siklini to'xtatadi va shu bilan polyubiquitylation faoliyatining faollashishiga yo'l qo'ymaydi. anafazani targ'ib qiluvchi kompleks (APC). SAC signaliga javob beradigan oqsillar quyidagilarni tashkil qiladi mitotik nazorat punkti kompleksi SAC oqsillarini o'z ichiga olgan (MCC), MAD2 /MAD3 (mitotik hibsga olish etishmovchiligi), BUB3 (benzimidazol tomonidan inhibe qilinmagan kurtak) va CDC20.[57] SAC tarkibiga kiradigan boshqa oqsillarni o'z ichiga oladi MAD1, BUB1, MPS1 va Avrora B. Yuqori eukaryotlar uchun SACning qo'shimcha regulyatorlari tarkibiga quyidagilar kiradi ROD-ZW10 kompleksi, p31kometa, XARITA, CDK1-siklin-B, NEK2 va PLK1.[58]

Tekshirish punktini faollashtirish
SAC noto'g'ri ulangan kinetoxorlar va shpindel o'rtasidagi o'zaro ta'sirni nazorat qiladi mikrotubulalar, va kinetoxorlar milga to'g'ri biriktirilguncha saqlanib qoladi. Davomida prometafaza, CDC20 va SAC oqsillari shpindel biriktirilishidan oldin kinetoxorlarda konsentratsiyalanadi. Ushbu oqsillar SAC ni ular chiqarilguncha va to'g'ri kinetoxora-mikrotubulalar biriktirilguncha faollashtiradi. Hatto bitta biriktirilmagan kinetoxora ham milni tekshirish punktini saqlab turishi mumkin.[57] Mikrotubula plyus uchlari biriktirilgandan va kinetoxora mikrotubulalari hosil bo'lgandan so'ng, MAD1 va MAD2 kinetoxora birikmasidan susayadi. Tekshirish punktini faollashtirishning yana bir regulyatori - kinetoxora tarangligi. Opa-singil kinetoxorlar qarama-qarshi shpindel qutblariga to'g'ri biriktirilganda, mitoz shpindagi kuchlar kinetoxorlarda kuchlanish hosil qiladi. Ikki tomonlama yo'naltirilgan opa-singil kinetoxorlar kinetoxora-mikrotubulalar birikmasini barqarorlashtiradi, zaif kuchlanish esa beqarorlashtiruvchi ta'sirga ega. Kabi noto'g'ri kinetoxor qo'shimchalariga javoban sintetik biriktirma, bu erda har ikkala kinetoxor ham bir milya qutbiga bog'lanib qoladi, hosil bo'lgan zaif taranglik noto'g'ri biriktirmani beqarorlashtiradi va kinetoxorni ish miliga to'g'ri o'rnatishga imkon beradi. Ushbu jarayon davomida mitoz shpindelga bog'langan, ammo kuchlanish ostida bo'lmagan kinetoxorlar shpindelning tekshiruv punktini ishga tushiradi. Aurora-B / Ipl1 kinazasi xromosoma yo'lovchi kompleksi noto'g'ri kinetoxor qo'shimchalaridagi kuchlanish sensori sifatida ishlaydi. U mikrotubulani ajratuvchi KINI kinesin MCAKni boshqarish orqali noto'g'ri qo'shimchalarni aniqlaydi va beqarorlashtiradi. DASH kompleksi, va Ndc80 / Hec1 murakkab[59] mikrotubule-kinetochore interfeysida.[58] Aurora-B / Ipl1 kinazasi ham tuzatishda juda muhimdir merotelic qo'shimchalar, bu erda bir kinetoxor bir vaqtning o'zida ikkala milning qutblariga biriktirilgan. Merotel qo'shimchalari etarlicha kuchlanish hosil qiladi va SAC tomonidan aniqlanmaydi va tuzatilmasdan, xromatid migratsiyasi tezligi tufayli xromosomalarning noto'g'ri ajratilishiga olib kelishi mumkin. SACni faollashtirish uchun mikrotubulalarni biriktirish mustaqil ravishda talab qilinsa-da, keskinlik SACning mustaqil regulyatori ekanligi noma'lum, garchi keskinlik bilan turli xil tartibga soluvchi xatti-harakatlar paydo bo'lishi aniq.
Faollashtirilgandan so'ng, milni tekshirish punkti bloklanadi anafaza inhibe qilish orqali kirish anafazani rivojlantiruvchi kompleks mitotik nazorat punkti faoliyatini tartibga solish orqali. APC ni mitotik nazorat punkti kompleksi tomonidan inhibe qilish mexanizmi juda yaxshi tushunilmagan, ammo MCC ning APC bilan a psevdosubstrat yordamida KEN-box motif BUBR1. Shu bilan birga, mitotik nazorat punkti kompleksi faollashtirilmoqda tsentromer oqsil CENP-E BUBR1 ni faollashtiradi, bu ham anafazani bloklaydi.[58]
Mitotik nazorat punktining kompleks shakllanishi
Mitotik nazorat punkti kompleksi tarkib topgan BUB3 bilan bog'langan MAD2 va MAD3 bilan birga CD20. MAD2 va MAD3 CDC20 da aniq bog'lanish joylariga ega va APC / C ni inhibe qilish uchun sinergik ta'sir ko'rsatadi. MAD3 kompleksi BUB3 dan tashkil topgan bo'lib, u Mad3 va BUB1B orqali qisqa chiziqli motif GLEBS motifi sifatida tanilgan. MCMni shakllantirish uchun amalga oshiriladigan qo'shimchalarning aniq tartibi noma'lum bo'lib qolmoqda. BUBR1-BUB3-Cdc20 boshqa kompleks hosil qilishi bilan bir vaqtning o'zida Mad2-Cdc20 kompleks hosil qilishi mumkin va natijada bu ikkita subkomplekslar mitotik nazorat punktlari kompleksini hosil qiladi.[57] Inson hujayralarida BUBR1 ning CDC20 bilan bog'lanishi MAD2 ning CDC20 bilan oldindan bog'lanishini talab qiladi, shuning uchun MAD2-CDC20 subkompleksi MCC hosil bo'lishining tashabbuskori vazifasini bajarishi mumkin. BUBR1 ning tükenmesi faqat Mad2-Cdc20 darajasining engil pasayishiga olib keladi, B2 esa BubR1-Bub3 ning Cdc20 bilan bog'lanishi uchun Mad2 kerak. Shunga qaramay, tekshiruv punktini faollashtirish uchun BUBR1 talab qilinadi.[58]
KMK hosil bo'lish mexanizmi noma'lum va kinetoxordan bog'liq va kinetoxordan mustaqil shakllanish uchun raqobatdosh nazariyalar mavjud. Kinetoxordan mustaqil nazariyani qo'llab-quvvatlash uchun MCC aniqlanadi S. cerevisiae yadro kinetokore birikmasi oqsillari mutatsiyaga uchragan hujayralar va SAC o'chirilgan hujayralar, bu mitoz paytida KMK ni kinetoxor lokalizatsiyasiz yig'ish mumkin degan fikrni bildiradi. Bir modelda biriktirilmagan prometafaza kinetoxorlari APCni ishlaydigan SAC orqali kinetoxorlarga jalb qilish orqali KPKni inhibisyoniga 'sezgirlashtirishi' mumkin. Bundan tashqari, turli xil SAC oqsillarining kamayishi natijasida MAD2 va BUBR1 ning kamayishi kinetoxordan mustaqil ravishda mitozning vaqtiga ta'sir qiladi, boshqa SAC oqsillarining etishmasligi esa mitozning davomiyligini o'zgartirmasdan disfunktsional SACga olib keladi. Shunday qilib, SAC ikki bosqichli taymer orqali ishlaydi, bu erda MAD2 va BUBR1 birinchi bosqichda mitozning davomiyligini nazorat qiladi, agar bu biriktirilmagan kinetoxorlar va boshqa SAC oqsillari bo'lsa, ikkinchi bosqichda uzaytirilishi mumkin.[58] Biroq, kinetoxordan mustaqil yig'ilishni yoqtirmaydigan dalillar mavjud. MCC davomida hali topilmadi interfaza, MCC esa uning tarkibidan kelib chiqmaydi X. laevis mayoz II yadro sperma qo'shilmagan ekstraktlar va nokodazol milni yig'ilishini oldini olish uchun.
MCC shakllanishining etakchi modeli "MAD2-shablon modeli" bo'lib, u MAD2 ni yaratish uchun MAD2 ning kinetoxora dinamikasiga bog'liq. MAD1 biriktirilmagan kinetoxorlarga joylashadi, shu bilan birga MAD2 bilan qattiq bog'lanadi. MAD2 va BubR1 ning kinetoxora lokalizatsiyasi ham bog'liq bo'lishi mumkin Aurora B kinazasi.[60] Aurora B etishmayotgan hujayralar xromosomalarda mikrotubulalar birikmasi bo'lmaganda ham metafazada ushlanib qolmaydi.[61] Biriktirilmagan kinetoxorlar avval MAD1-C-MAD2-p31 bilan bog'lanadikometa murakkab va p31-ni chiqaradikometa noma'lum mexanizmlar orqali. Natijada paydo bo'lgan MAD-C-MAD2 kompleksi Mad2 (O-Mad2) ning ochiq konformerini kinetoxorlarga jalb qiladi. Ushbu O-Mad2 konformatsiyasini yopiq Mad2 (C-Mad2) ga o'zgartiradi va Mad1 ni bog'laydi. Ushbu Mad1 / C-Mad2 kompleksi kinetoxorlarga ko'proq O-Mad2 ni jalb qilish uchun javobgardir, bu uning konformatsiyasini C-Mad2 ga o'zgartiradi va Cdc20 ni avto-amplifikatsiya reaktsiyasida bog'laydi. MAD1 va CDC20 ikkalasida ham o'xshash MAD2 bog'lash motifi mavjud bo'lgani uchun, bo'sh O-MAD2 konformatsiyasi CDC20 bilan bog'lanishda C-MAD2 ga o'zgaradi. Bu ijobiy teskari aloqa davri p31 tomonidan salbiy tartibga solinadikometa, bu raqobatdosh ravishda MAD1 yoki CDC20 bilan bog'langan C-MAD2 ga ulanadi va keyingi O-MAD2 ning C-MAD2 bilan bog'lanishini kamaytiradi. P31-ni hisobga olgan holda qo'shimcha boshqaruv mexanizmlari ham mavjud bo'lishi mumkinkometa pastki eukaryotlarda mavjud emas. Shunday qilib "shablon modeli" nomenklaturasi MAD1-C-MAD2 C-MAD2-CDC20 nusxalarini shakllantirish uchun shablon vazifasini bajaradigan jarayondan kelib chiqadi. Cdc20 ning bu sekvestratsiyasi shpindelni tekshirish punktini saqlash uchun juda muhimdir.[57]
Tekshirish nuqtasini o'chirish
To'g'ri ikki yo'naltirilganidan so'ng SACni o'chirish uchun bir nechta mexanizmlar mavjud opa-singil xromatidlar. Mikrotubula-kinetoxor biriktirilganda, a orqali tozalash mexanizmi dynein-dynein motor kompleksi shpindel tekshiruv punkti oqsillarini kinetoxordan uzoqroqqa tashiydi.[58] MAD1, MAD2, MPS1 va tarkibiga kiradigan tozalangan oqsillar CENP-F, keyin qayta taqsimlanadi shpindel ustunlari. Yalang'ochlash jarayoni shikastlanmagan mikrotubulalar tuzilishiga, shuningdek mikrotubulalar bo'ylab dynein harakatiga juda bog'liq. Shuningdek, C-MAD2 ijobiy teskari aloqa davri regulyatori sifatida ishlaydi, p31kometa shuningdek, SAC deaktivatori vazifasini bajarishi mumkin. Bog'lanmagan kinetoxoralar vaqtincha p31 faolsizlantiradikometa, ammo biriktirilishi oqsilni qayta faollashtiradi va MAD2 aktivatsiyasini inhibe qiladi, ehtimol inhibitoryal fosforillanish bilan. SAC inaktivatsiyasining yana bir mumkin bo'lgan mexanizmi, MAD2-CDC20 kompleksining energiyaga bog'liq bo'lgan dissotsiatsiyasi natijasida CDC20 ning degradativ bo'lmagan hamma joyda birlashishi natijasida yuzaga keladi. Aksincha, hamma joyda yo'q qilinadigan ferment himoyalash SACni saqlash uchun talab qilinadi. Shunday qilib, biriktirilmagan kinetoxorlar MAD2-CDC20 subkompleksini uning tarkibiy qismlaridan doimiy ravishda qayta tiklash orqali nazorat punktini saqlab turishadi. SAC, shuningdek, APC faollashishi natijasida o'chirilishi mumkin proteoliz. SAC anafaza paytida singil-xromatid birlashuvining yo'qolishi bilan qayta faollashmaganligi sababli, B siklinining proteolizasi va CDK1-siklin-B kinazaning inaktivatsiyasi ham SAC faolligini inhibe qiladi. Anafaza paytida MPS1 ning parchalanishi singan-xromatid birlashmasi chiqarilgandan so'ng SAC ning qayta faollashuviga to'sqinlik qiladi. Tekshirish punkti o'chirilgandan so'ng va hujayra tsiklining normal anafazasi paytida anafazani ko'taruvchi kompleks MCC faolligini pasayishi bilan faollashadi. Bu sodir bo'lganda fermentlar kompleksi poliubikvitinatlar anafaza inhibitori sekurin. Metafaza oxirida sekurinning hamma joyda tarqalishi va yo'q bo'lib ketishi natijasida separaz deb ataladigan faol proteaz ajralib chiqadi. Separaza anafazani faollashtirish uchun opa-singil xromatidlarni ushlab turuvchi birlashma molekulalarini ajratib turadi.[23]
SACni o'chirish uchun yangi model S. cerevisiae: mexanik kalit
Kinetoxordagi mikrotubulalarni biriktirilishi SAC signalizatsiyasining aniq bosqichlarini qanday buzishi mumkinligini tushuntirish uchun yangi mexanizm taklif qilindi. Bog'lanmagan kinetoxorada MCC hosil bo'lishining birinchi bosqichi Spc105 ni kinaz Mps1 bilan fosforillashi. Keyin fosforillangan Spc105 Bub1 va 3 ning quyi qismida signal beruvchi oqsillarni yollashga qodir; Mad 1,2 va 3; va CD20. Biriktirilmagan kinetoxorlarda Mad1 bilan bog'lanish, Mad2 ning konformatsion o'zgarishiga olib keladi, bu uni ochiq shakldan (O-Mad2) yopiq shaklga (C-Mad2.) Aylantiradi, keyin Mad1 bilan bog'langan C-Mad2 ikkinchi O-Mad2 bilan kamayadi. va Cdc20 atrofida yopilishini katalizlaydi. Ushbu C-Mad2 va Cdc20 kompleksi, MCC, Mad1 va C-Mad2 ni kinetoxorda qoldirib, boshqa MCC hosil qiladi. MCClar har biri APC / C bilan o'zaro ta'sirini oldini olish uchun ikkita Cdc20 molekulasini ajratadi va shu bilan SACni saqlab qoladi.[23] Spps105 ning Mps1 ning fosforillanishi SAC signalizatsiya yo'lini boshlash uchun zarur va etarli, ammo bu qadam faqat kinetoxoraga mikrotubulalar biriktirilmagan holda sodir bo'lishi mumkin. Endogen Mps1 xromosomadan uzoqda joylashgan tashqi kinetoxor mintaqasida joylashgan Ndc80 ning kalponin-homologiyasi (CH) domeni bilan bog'langanligi ko'rsatilgan. Mps1 tashqi kinetoxorga joylashtirilgan bo'lsa ham, Ndc80-da egiluvchan menteşe mintaqalari tufayli ichki kinetoxor va Spc105 fosforilat ichida lokalizatsiya qilish imkoniyatiga ega. Shu bilan birga, mexanik kalit modeli kinetoxorga mikrotubulani oxirigacha bog'lab qo'yish SACni ikkita mexanizm orqali o'chirishni taklif qiladi. Biriktirilgan mikrotubulaning mavjudligi Ndc80 CH domeni va Spc105 orasidagi masofani oshiradi. Bundan tashqari, Dam1 / DASH, biriktirilgan mikrotubula atrofida halqa hosil qiluvchi 160 ta oqsildan iborat katta kompleks, ikkita oqsil o'rtasida to'siq bo'lib xizmat qiladi. Ajratish Mps1 va Spc105 o'rtasidagi o'zaro ta'sirni oldini oladi va shu bilan SAC signalizatsiya yo'lini inhibe qiladi.[62]
Shuni ta'kidlash kerakki, ushbu model yuqori darajadagi organizmlarda, shu jumladan hayvonlarda SACni tartibga solishda qo'llanilmaydi. Mexanik almashtirish mexanizmining asosiy jihati shundaki S. cerevisiae kinetoxoraning tuzilishi faqat bitta mikrotubulani biriktirishga imkon beradi. Boshqa tomondan, hayvonlardagi kinetoxorlar juda ko'p miqdordagi mikrotubulalar uchun bog'lanish joylarini o'z ichiga olgan juda murakkab mashlardir.[63] Kinetoxorni bog'laydigan joylarida mikrotubulani biriktirish SACni o'chirish va anafazaga o'tish uchun zarur emas. Shuning uchun mikrotubulaga bog'langan va mikrotubulaga biriktirilmagan holatlar hayvonlarning kinetoxorasida SAC inhibe qilingan holda birga yashaydi. Ushbu model biriktirilgan kinetoxora bilan bog'liq bo'lgan Mps1-ni qo'shni biriktirilmagan kinetoxorda Spc105-ni fosforlashidan saqlaydigan to'siqni o'z ichiga olmaydi. Bundan tashqari, xamirturushli Dam1 / DASH kompleksi hayvon hujayralarida mavjud emas.
Milya tekshiruv punktidagi nuqsonlar va saraton
Ish milini nazorat qilish punkti noto'g'ri ishlaganda, bu xromosomalarning noto'g'ri taqsimlanishiga olib kelishi mumkin, aneuploidiya va hatto shish paydo bo'lishi.[58] Transformatsiya genomik yaxlitlikni saqlash, ayniqsa butun xromosomalarning yoki ularning katta qismlarining yalpi darajasida buzilganda sodir bo'ladi va tezlashadi. Darhaqiqat, aneuploidiya odamning qattiq o'smalarining eng keng tarqalgan xususiyati hisoblanadi va shu sababli shpindelni yig'ish punkti o'smalarga qarshi terapiyaning mumkin bo'lgan maqsadi sifatida qaralishi mumkin.[64] Bu juda kam baholangan haqiqat, chunki ma'lum genlardagi mutatsiyalar ma'lum onkogenlar yoki o'simta supressori birinchi navbatda genetik beqarorlik va shish paydo bo'lishining orqasida deb o'ylashadi. Odatda hujayra tsiklidagi turli xil nazorat punktlari genomik yaxlitlikka g'amxo'rlik qiladi, chunki ular uyali gomeostazni saqlab turish va shish paydo bo'lishining oldini olish uchun juda muhimdir. Har xil hujayra siklida xromosomalarning beqarorligini (CIN) oldini oluvchi xromosomalarning to'g'ri ajratilishini ta'minlash uchun bir nechta shpindelni yig'ish punkti oqsillari ham ijobiy, ham salbiy regulyator sifatida ishlaydi. genomning beqarorligi.

Hozirgi vaqtda genomik yaxlitlik bir necha darajalarda qadrlanadi, bu erda ba'zi o'smalar beqarorlikni namoyon qiladi, ular asos o'rnini bosish, qo'shimchalar va o'chirishlar bilan namoyon bo'ladi, aksariyat qismi esa butun xromosomalarning yutuqlarini yoki yo'qotishlarini ko'rsatadi.[65]
Mitotik regulyatsion oqsillarning o'zgarishi aneuploidiyaga olib kelishi mumkinligi va bu tez-tez yuz beradigan hodisa saraton,[66] dastlab bu genlar saraton to'qimalarida mutatsiyaga uchrashi mumkin deb o'ylashgan edi.[67]
Saraton kasalliklarida mutatsiyaga uchragan genlar
In some cancers the genes that underlie the defects resulting in transformation are well characterized. In the hematological cancers such as multiple myeloma cytogenetic abnormalities are very common due to the inherent nature of DNA breaks needed for immunoglobulin gene rearrangement. However, defects in proteins such as MAD2 that function predominantly at the SAC also are characterized in multiple myeloma.[68] Most solid tumors are also predominantly aneuploid. For colorectal cancer, BUB1 and BUBR1 and amplification of STK15 are key regulators that have been implicated in the genomic instability resulting in cancer.[69] In breast cancer, the genetic form characterized by the BRCA-1 gene exhibits greater levels of genomic instability than sporadic forms. Experiments showed that BRCA-1 null mice have decreased expression of the key spindle checkpoint protein MAD2 .[70] For other cancers, more work is warranted to identify the causes of aneuploidy.
Other genes not traditionally associated with the SAC in cancer
Clearly variations in the physiological levels of these proteins (such as Mad2 or BubR1) are associated with aneuploidy and tumorigenesis, and this has been demonstrated using hayvon modellari.[71][72] However, recent studies indicate that what seems to happen is a more complicated scenario: aneuploidy would drive a high incidence of tumorigenesis only when alterations in the levels of specific mitotic checkpoint components (either reduction or overexpression) in tissues is also inducing other defects able to predispose them to tumors.[73]That is, defects such as an increase in DNA damage, chromosomal rearrangements, and/or a decreased incidence of cell death. For some mitotic checkpoint components, it is known that they are implicated in functions outside mitosis: nuclear import (Mad1), transcriptional repression (Bub3), and cell death, DNA damage response, aging, and megakaryopoiesis for BubR1. All this supports the conclusion that increase in tumorigenesis is associated with defects other than aneuploidy alone.[73]
Cancer-associated mutations affecting known checkpoint genes like BUB1 or BUBR1 are actually rare. However, several proteins implicated in cancer have intersections to spindle assembly networks. Key tumor suppressors such as p53 also play a role in the spindle checkpoint. Absence of p53, the most commonly mutated gene in human cancer, has a major effect on cell cycle checkpoint regulators and has been shown to act at the G1 checkpoint in the past, but now appears to be important in regulating the spindle checkpoint as well.[74] Another key aspect of cancer is inhibition of cell death or apoptoz. Survivin, a member of the inhibitor of apoptosis (IAP) family, is localized in pools at microtubules of the mitotic spindle near the centrosomes and at the kinetochores of metaphase chromosomes. Not only does survivin inhibit apoptosis to promote tumorigenesis, but it has been implicated (through experimental knockout mice) as an important regulator of chromosome segregation, and late stage mitosis similar to its role in more primitive organisms.[75]
Other aspects of the spindle assembly checkpoint such as kinetochore attachment, microtubule function, and sister chromatid cohesion are likely to be defective as well to cause aneuploidy. Cancer cells have been observed to divide in multiple directions by evading the spindle assembly checkpoint resulting in multipolar mitoses.[76] The multipolar metaphase-anaphase transition occurs through an incomplete separase cycle that results in frequent nondisjunction events which amplify aneuploidy in cancer cells.
SAC cancer therapies

Advances in this field have led to the introduction of development of some therapies targeted at spindle assembly defects. Older treatments such as vinca alkaloids and taxanes target microtubules that accompany mitotic spindle formation via disruption of microtubule dynamics which engage the SAC arresting the cell and eventually leading to its death.[77] taksol va Detsetaksel both are still used in the treatment of breast cancer, ovarian cancer and other types of epithelial cancer. However, these treatments are often characterized by high rates of side effects and drug resistance.
Other targets within the network of regulators that influence the SAC are also being pursued; strong interest has shifted towards the aurora kinase oqsillar.[78] The kinase gene Avrora A when amplified acts as an oncogene overriding the SAC leading to abnormal initiation of anaphase and subsequent aneuploidy and also resistance to TAXOL .[79] Excitingly, a small molecule inhibitor of Aurora A has shown antitumor effects in an in vivo model suggesting that this might be a good target for further clinical development.[80] Avrora B inhibitors, which are also in clinical development lead to abnormal kinetochore to microtubule attachment and abrogate the mitotic checkpoint as well.[78] Survivin is also an attractive molecular target for clinical therapeutic development as it acts as a major node in a multitude of pathways, one of which is spindle formation and checkpoint control.[81] Even further approaches have included a look at inhibition of mitotic motor proteins like KSP. These inhibitors, which have recently entered clinical trials, cause mitotic arrest and by engaging the spindle assembly checkpoint and induce apoptosis.[82][3]
Adabiyotlar
- ^ Santaguida S, Musacchio A (September 2009). "Kinetoxoralarning hayoti va mo''jizalari". EMBO jurnali. 28 (17): 2511–31. doi:10.1038 / emboj.2009.173. PMC 2722247. PMID 19629042.
- ^ Morgan, David Owen, 1958- (2007). Hujayra aylanishi: boshqarish tamoyillari. London: New Science Press. ISBN 978-0-19-920610-0. OCLC 70173205.CS1 maint: bir nechta ism: mualliflar ro'yxati (havola)
- ^ a b Sinha, D.; Duijf, P.H.G.; Xanna, K.K. (2019), "Mitotic slippage: an old tale with a new twist", Hujayra aylanishi, 18 (1): 7–15, doi:10.1080/15384101.2018.1559557, PMC 6343733, PMID 30601084
- ^ Santaguida S, Amon A (August 2015). "Short- and long-term effects of chromosome mis-segregation and aneuploidy". Molekulyar hujayra biologiyasining tabiat sharhlari. 16 (8): 473–85. doi:10.1038/nrm4025. hdl:1721.1/117201. PMID 26204159.
- ^ a b Zirkle RE (March 1970). "Ultraviolet-microbeam irradiation of newt-cell cytoplasm: spindle destruction, false anaphase, and delay of true anaphase". Radiatsion tadqiqotlar. 41 (3): 516–37. Bibcode:1970RadR...41..516Z. doi:10.2307/3572841. JSTOR 3572841. PMID 5438206.
- ^ Rieder CL, Palazzo RE (July 1992). "Colcemid and the mitotic cycle". Hujayra fanlari jurnali. 102 ( Pt 3) (3): 387–92. PMID 1506421.
- ^ Burke DJ, Stukenberg PT (April 2008). "Linking kinetochore-microtubule binding to the spindle checkpoint". Rivojlanish hujayrasi. 14 (4): 474–9. doi:10.1016/j.devcel.2008.03.015. PMC 2696048. PMID 18410725.
- ^ a b Li R, Murray AW (August 1991). "Feedback control of mitosis in budding yeast". Hujayra. 66 (3): 519–31. doi:10.1016/0092-8674(81)90015-5. PMID 1651172.
- ^ Hoyt MA, Totis L, Roberts BT (August 1991). "S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function". Hujayra. 66 (3): 507–17. doi:10.1016/0092-8674(81)90014-3. PMID 1651171.
- ^ Neff MW, Burke DJ (September 1992). "A delay in the Saccharomyces cerevisiae cell cycle that is induced by a dicentric chromosome and dependent upon mitotic checkpoints". Molekulyar va uyali biologiya. 12 (9): 3857–64. doi:10.1128/MCB.12.9.3857. PMC 360258. PMID 1324407.
- ^ Wells WA, Murray AW (April 1996). "Aberrantly segregating centromeres activate the spindle assembly checkpoint in budding yeast". Hujayra biologiyasi jurnali. 133 (1): 75–84. doi:10.1083/jcb.133.1.75. PMC 2120768. PMID 8601615.
- ^ Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW (August 1996). "Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption". Ilm-fan. 273 (5277): 953–6. Bibcode:1996Sci...273..953H. doi:10.1126/science.273.5277.953. PMID 8688079.
- ^ Wang Y, Burke DJ (December 1995). "Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae". Molekulyar va uyali biologiya. 15 (12): 6838–44. doi:10.1128/MCB.15.12.6838. PMC 230938. PMID 8524250.
- ^ Spencer F, Hieter P (October 1992). "Centromere DNA mutations induce a mitotic delay in Saccharomyces cerevisiae". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 89 (19): 8908–12. Bibcode:1992PNAS...89.8908S. doi:10.1073/pnas.89.19.8908. JSTOR 2360300. PMC 50033. PMID 1409584.
- ^ Hardwick KG, Li R, Mistrot C, Chen RH, Dann P, Rudner A, Murray AW (June 1999). "Lesions in many different spindle components activate the spindle checkpoint in the budding yeast Saccharomyces cerevisiae". Genetika. 152 (2): 509–18. PMC 1460633. PMID 10353895.
- ^ McIntosh JR (1991). "Structural and mechanical control of mitotic progression". Kantitativ biologiya bo'yicha sovuq bahor porti simpoziumlari. 56: 613–9. doi:10.1101/sqb.1991.056.01.070. PMID 1819511.
- ^ a b Rieder CL, Cole RW, Khodjakov A, Sluder G (August 1995). "The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores". Hujayra biologiyasi jurnali. 130 (4): 941–8. doi:10.1083/jcb.130.4.941. PMC 2199954. PMID 7642709.
- ^ Li X, Nicklas RB (March 1997). "Tension-sensitive kinetochore phosphorylation and the chromosome distribution checkpoint in praying mantid spermatocytes". Hujayra fanlari jurnali. 110 ( Pt 5) (5): 537–45. PMID 9092936.
- ^ Rieder CL, Khodjakov A, Paliulis LV, Fortier TM, Cole RW, Sluder G (May 1997). "Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 94 (10): 5107–12. Bibcode:1997PNAS...94.5107R. doi:10.1073/pnas.94.10.5107. PMC 24639. PMID 9144198.
- ^ Conlon I, Raff M (January 1999). "Size control in animal development". Hujayra. 96 (2): 235–44. doi:10.1016/S0092-8674(00)80563-2. PMID 9988218.
- ^ Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA (June 1999). "Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A". Tabiat hujayralari biologiyasi. 1 (2): 88–93. doi:10.1038/10054. PMID 10559879.
- ^ Mayor T, Meraldi P, Stierhof YD, Nigg EA, Fry AM (June 1999). "Protein kinases in control of the centrosome cycle". FEBS xatlari. 452 (1–2): 92–5. doi:10.1016/S0014-5793(99)00534-7. PMID 10376685.
- ^ a b v Morgan, David O. (2006-09-06). The Cell Cycle: Principles of Control (Primers in Biology) (1 nashr). New Science Press, Ltd. ISBN 978-0-87893-508-6.
- ^ Nicklas RB (January 1997). "How cells get the right chromosomes". Ilm-fan. 275 (5300): 632–7. doi:10.1126/science.275.5300.632. PMID 9005842.
- ^ Loncarek J, Kisurina-Evgenieva O, Vinogradova T, Hergert P, La Terra S, Kapoor TM, Khodjakov A (November 2007). "The centromere geometry essential for keeping mitosis error free is controlled by spindle forces". Tabiat. 450 (7170): 745–9. Bibcode:2007Natur.450..745L. doi:10.1038/nature06344. PMC 2586812. PMID 18046416.
- ^ Dewar H, Tanaka K, Nasmyth K, Tanaka TU (March 2004). "Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle". Tabiat. 428 (6978): 93–7. Bibcode:2004Natur.428...93D. doi:10.1038/nature02328. PMID 14961024.
- ^ Cimini D, Wan X, Hirel CB, Salmon ED (September 2006). "Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors". Hozirgi biologiya. 16 (17): 1711–8. doi:10.1016/j.cub.2006.07.022. PMID 16950108.
- ^ Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Gandara DR (March 2008). "Aurora kinases as anticancer drug targets". Klinik saraton tadqiqotlari. 14 (6): 1639–48. doi:10.1158/1078-0432.CCR-07-2179. PMID 18347165.
- ^ a b Hirano T (2000). "Chromosome cohesion, condensation, and separation". Biokimyo fanining yillik sharhi. 69: 115–44. doi:10.1146/annurev.biochem.69.1.115. PMID 10966455.
- ^ Tanaka K, Hao Z, Kai M, Okayama H (October 2001). "Establishment and maintenance of sister chromatid cohesion in fission yeast by a unique mechanism". EMBO jurnali. 20 (20): 5779–90. doi:10.1093/emboj/20.20.5779. PMC 125673. PMID 11598020.
- ^ Gonzalez C, Casal Jimenez J, Ripoll P, Sunkel CE (January 1991). "The spindle is required for the process of sister chromatid separation in Drosophila neuroblasts". Eksperimental hujayra tadqiqotlari. 192 (1): 10–5. doi:10.1016/0014-4827(91)90150-S. PMID 1898588.
- ^ Losada A, Hirano T (October 2001). "Shaping the metaphase chromosome: coordination of cohesion and condensation". BioEssays. 23 (10): 924–35. doi:10.1002/bies.1133. PMID 11598959.
- ^ Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC (December 2001). "Requirement of heterochromatin for cohesion at centromeres". Ilm-fan. 294 (5551): 2539–42. Bibcode:2001Sci...294.2539B. doi:10.1126/science.1064027. PMID 11598266.
- ^ Nonaka N, Kitajima T, Yokobayashi S, Xiao G, Yamamoto M, Grewal SI, Watanabe Y (January 2002). "Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast". Tabiat hujayralari biologiyasi. 4 (1): 89–93. doi:10.1038/ncb739. PMID 11780129.
- ^ Hall IM, Noma K, Grewal SI (January 2003). "RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 100 (1): 193–8. Bibcode:2003PNAS..100..193H. doi:10.1073/pnas.232688099. PMC 140924. PMID 12509501.
- ^ Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, Nakayama T, Oshimura M (August 2004). "Dicer is essential for formation of the heterochromatin structure in vertebrate cells". Tabiat hujayralari biologiyasi. 6 (8): 784–91. doi:10.1038/ncb1155. PMID 15247924.
- ^ Weber SA, Gerton JL, Polancic JE, DeRisi JL, Koshland D, Megee PC (September 2004). "The kinetochore is an enhancer of pericentric cohesin binding". PLOS biologiyasi. 2 (9): E260. doi:10.1371/journal.pbio.0020260. PMC 490027. PMID 15309047.

- ^ Prasanth SG, Prasanth KV, Siddiqui K, Spector DL, Stillman B (July 2004). "Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance". EMBO jurnali. 23 (13): 2651–63. doi:10.1038/sj.emboj.7600255. PMC 449767. PMID 15215892.
- ^ Shimada K, Gasser SM (January 2007). "The origin recognition complex functions in sister-chromatid cohesion in Saccharomyces cerevisiae". Hujayra. 128 (1): 85–99. doi:10.1016/j.cell.2006.11.045. PMID 17218257.
- ^ Kato H, Matsunaga F, Miyazaki S, Yin L, D'Urso G, Tanaka K, Murakami Y (April 2008). "Schizosaccharomyces pombe Orc5 plays multiple roles in the maintenance of genome stability throughout the cell cycle". Hujayra aylanishi. 7 (8): 1085–96. doi:10.4161/cc.7.8.5710. PMID 18414064.
- ^ Hauf S, Watanabe Y (October 2004). "Kinetochore orientation in mitosis and meiosis". Hujayra. 119 (3): 317–27. doi:10.1016/j.cell.2004.10.014. PMID 15507205.
- ^ Sonoda E, Matsusaka T, Morrison C, Vagnarelli P, Hoshi O, Ushiki T, Nojima K, Fukagawa T, Waizenegger IC, Peters JM, Earnshaw WC, Takeda S (December 2001). "Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells". Rivojlanish hujayrasi. 1 (6): 759–70. doi:10.1016/S1534-5807(01)00088-0. PMID 11740938.
- ^ Vass S, Cotterill S, Valdeolmillos AM, Barbero JL, Lin E, Warren WD, Heck MM (February 2003). "Depletion of Drad21/Scc1 in Drosophila cells leads to instability of the cohesin complex and disruption of mitotic progression" (PDF). Hozirgi biologiya. 13 (3): 208–18. doi:10.1016/S0960-9822(03)00047-2. PMID 12573216.
- ^ Haering CH, Löwe J, Hochwagen A, Nasmyth K (April 2002). "Molecular architecture of SMC proteins and the yeast cohesin complex". Molekulyar hujayra. 9 (4): 773–88. doi:10.1016/S1097-2765(02)00515-4. PMID 11983169.
- ^ Hirano T (January 1999). "SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates?". Genlar va rivojlanish. 13 (1): 11–9. doi:10.1101/gad.13.1.11. PMID 9887095.
- ^ Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K (June 1998). "An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast". Hujayra. 93 (6): 1067–76. doi:10.1016/S0092-8674(00)81211-8. PMID 9635435.
- ^ Alexandru G, Uhlmann F, Mechtler K, Poupart MA, Nasmyth K (May 2001). "Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast". Hujayra. 105 (4): 459–72. doi:10.1016/S0092-8674(01)00362-2. PMID 11371343.
- ^ Leismann O, Herzig A, Heidmann S, Lehner CF (September 2000). "Degradation of Drosophila PIM regulates sister chromatid separation during mitosis". Genlar va rivojlanish. 14 (17): 2192–205. doi:10.1101/gad.176700. PMC 316890. PMID 10970883.
- ^ Zur A, Brandeis M (February 2001). "Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis". EMBO jurnali. 20 (4): 792–801. doi:10.1093/emboj/20.4.792. PMC 145417. PMID 11179223.
- ^ Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM (November 2000). "Characterization of vertebrate cohesin complexes and their regulation in prophase". Hujayra biologiyasi jurnali. 151 (4): 749–62. doi:10.1083/jcb.151.4.749. PMC 2169443. PMID 11076961.
- ^ Losada A, Yokochi T, Kobayashi R, Hirano T (August 2000). "Identification and characterization of SA/Scc3p subunits in the Xenopus and human cohesin complexes". Hujayra biologiyasi jurnali. 150 (3): 405–16. doi:10.1083/jcb.150.3.405. PMC 2175199. PMID 10931856.
- ^ Giménez-Abián JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters JM (July 2004). "Regulation of sister chromatid cohesion between chromosome arms". Hozirgi biologiya. 14 (13): 1187–93. doi:10.1016/j.cub.2004.06.052. PMID 15242616.
- ^ Paliulis LV, Nicklas RB (December 2004). "Micromanipulation of chromosomes reveals that cohesion release during cell division is gradual and does not require tension". Hozirgi biologiya. 14 (23): 2124–9. doi:10.1016/j.cub.2004.11.052. PMID 15589155.
- ^ Nakajima M, Kumada K, Hatakeyama K, Noda T, Peters JM, Hirota T (December 2007). "The complete removal of cohesin from chromosome arms depends on separase". Hujayra fanlari jurnali. 120 (Pt 23): 4188–96. doi:10.1242/jcs.011528. PMID 18003702.
- ^ McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K (March 2005). "Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells". PLOS biologiyasi. 3 (3): e86. doi:10.1371/journal.pbio.0030086. PMC 1054882. PMID 15737064.

- ^ Salic A, Waters JC, Mitchison TJ (September 2004). "Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis". Hujayra. 118 (5): 567–78. doi:10.1016/j.cell.2004.08.016. PMID 15339662.
- ^ a b v d De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, Musacchio A (February 2005). "Mad1 / Mad2 kompleksi shpindelni yig'ish punktida Mad2 aktivatsiyasi uchun shablon sifatida". Hozirgi biologiya. 15 (3): 214–25. doi:10.1016 / j.cub.2005.01.038. PMID 15694304.
- ^ a b v d e f g Musacchio A, Salmon ED (May 2007). "The spindle-assembly checkpoint in space and time". Tabiat sharhlari. Molekulyar hujayra biologiyasi. 8 (5): 379–93. doi:10.1038/nrm2163. PMID 17426725.
- ^ Martin-Lluesma S, Stucke VM, Nigg EA (September 2002). "Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2". Ilm-fan. 297 (5590): 2267–70. Bibcode:2002Sci...297.2267M. doi:10.1126/science.1075596. PMID 12351790.
- ^ Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH (June 2003). "Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension". EMBO jurnali. 22 (12): 2934–47. doi:10.1093/emboj/cdg307. PMC 162159. PMID 12805209.
- ^ Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM (April 2003). "Hesperadin kichik molekulasi Aurora B-ning kinetoxor-mikrotubulalarning biriktirilishini to'g'rilashda va shpindellarni yig'ish punktini saqlashdagi rolini ochib beradi". Hujayra biologiyasi jurnali. 161 (2): 281–94. doi:10.1083 / jcb.200208092. PMC 2172906. PMID 12707311.
- ^ Aravamudhan P, Goldfarb AA, Joglekar AP (July 2015). "The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling". Tabiat hujayralari biologiyasi. 17 (7): 868–79. doi:10.1038/ncb3179. PMC 4630029. PMID 26053220.
- ^ Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). Molecular Biology of The Cell (6th ed.). New York, NY: Garland Science, Taylor & Francis Group. p. 988. ISBN 978-0-8153-4432-2.
- ^ Kops GJ, Weaver BA, Cleveland DW (October 2005). "On the road to cancer: aneuploidy and the mitotic checkpoint". Tabiat sharhlari. Saraton. 5 (10): 773–85. doi:10.1038/nrc1714. PMID 16195750.
- ^ Lengauer C, Kinzler KW, Vogelstein B (December 1998). "Genetic instabilities in human cancers". Tabiat. 396 (6712): 643–9. Bibcode:1998Natur.396..643L. doi:10.1038/25292. PMID 9872311.
- ^ Weaver BA, Cleveland DW (December 2006). "Does aneuploidy cause cancer?". Hujayra biologiyasidagi hozirgi fikr. 18 (6): 658–67. doi:10.1016/j.ceb.2006.10.002. PMID 17046232.
- ^ Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B (March 1998). "Odam saraton kasalligida mitotik tekshiruv punkti genlarining mutatsiyalari". Tabiat. 392 (6673): 300–3. Bibcode:1998Natur.392..300C. doi:10.1038/32688. PMID 9521327.
- ^ Díaz-Rodríguez E, Álvarez-Fernández S, Chen X, Paiva B, López-Pérez R, García-Hernández JL, San Miguel JF, Pandiella A (2011). "Deficient spindle assembly checkpoint in multiple myeloma". PLOS One. 6 (11): e27583. Bibcode:2011PLoSO...627583D. doi:10.1371/journal.pone.0027583. PMC 3223182. PMID 22132115.

- ^ Grady, William M. (2004). "Genomic instability and colon cancer". Saraton va metastaz bo'yicha sharhlar. 23 (1–2): 11–27. doi:10.1023/A:1025861527711. PMID 15000146.
- ^ Wang RH, Yu H, Deng CX (December 2004). "A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 101 (49): 17108–13. Bibcode:2004PNAS..10117108W. doi:10.1073/pnas.0407585101. PMC 535394. PMID 15563594.
- ^ Sotillo R, Hernando E, Díaz-Rodríguez E, Teruya-Feldstein J, Cordón-Cardo C, Lowe SW, Benezra R (January 2007). "Mad2 overexpression promotes aneuploidy and tumorigenesis in mice". Saraton xujayrasi. 11 (1): 9–23. doi:10.1016/j.ccr.2006.10.019. PMC 1850996. PMID 17189715.
- ^ Yamamoto Y, Matsuyama H, Chochi Y, Okuda M, Kawauchi S, Inoue R, Furuya T, Oga A, Naito K, Sasaki K (April 2007). "Overexpression of BUBR1 is associated with chromosomal instability in bladder cancer". Saraton genetikasi va sitogenetikasi. 174 (1): 42–7. doi:10.1016/j.cancergencyto.2006.11.012. PMID 17350465.
- ^ a b Weaver BA, Cleveland DW (June 2009). "The role of aneuploidy in promoting and suppressing tumors". Hujayra biologiyasi jurnali. 185 (6): 935–7. doi:10.1083/jcb.200905098. PMC 2711620. PMID 19528293.
- ^ Cross, Shawn M.; Sanchez, Carissa A; Morgan, Ketrin A.; Schimke, Melana K.; Reid, Brian J. (1995). "A p53-dependant mouse spindle checkpoint". Ilm-fan. 3 (5202): 1353–1356. Bibcode:1995Sci...267.1353C. doi:10.1126/science.7871434. PMID 7871434.
- ^ Altieri DC (December 2001). "The molecular basis and potential role of survivin in cancer diagnosis and therapy". Molekulyar tibbiyot tendentsiyalari. 7 (12): 542–7. doi:10.1016/S1471-4914(01)02243-2. PMID 11733216.
- ^ Gisselsson D, Håkanson U, Stoller P, Marti D, Jin Y, Rosengren AH, Stewénius Y, Kahl F, Panagopoulos I (April 2008). "When the genome plays dice: circumvention of the spindle assembly checkpoint and near-random chromosome segregation in multipolar cancer cell mitoses". PLOS One. 3 (4): e1871. Bibcode:2008PLoSO...3.1871G. doi:10.1371/journal.pone.0001871. PMC 2289843. PMID 18392149.

- ^ Zhou J, Giannakakou P (January 2005). "Targeting microtubules for cancer chemotherapy". Hozirgi dorivor kimyo. Saratonga qarshi vositalar. 5 (1): 65–71. doi:10.2174/1568011053352569. PMID 15720262.
- ^ a b Carvajal RD, Tse A, Schwartz GK (December 2006). "Aurora kinases: new targets for cancer therapy". Klinik saraton tadqiqotlari. 12 (23): 6869–75. doi:10.1158/1078-0432.CCR-06-1405. PMID 17145803.
- ^ Anand S, Penrhyn-Lowe S, Venkitaraman AR (January 2003). "AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol". Saraton xujayrasi. 3 (1): 51–62. doi:10.1016/S1535-6108(02)00235-0. PMID 12559175.
- ^ Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM (March 2004). "VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo". Tabiat tibbiyoti. 10 (3): 262–7. doi:10.1038/nm1003. PMID 14981513.
- ^ Altieri DC (January 2008). "Survivin, cancer networks and pathway-directed drug discovery". Tabiat sharhlari. Saraton. 8 (1): 61–70. doi:10.1038/nrc2293. PMID 18075512.
- ^ Tao W, South VJ, Zhang Y, Davide JP, Farrell L, Kohl NE, Sepp-Lorenzino L, Lobell RB (July 2005). "Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage". Saraton xujayrasi. 8 (1): 49–59. doi:10.1016/j.ccr.2005.06.003. PMID 16023598.
Qo'shimcha o'qish
- Larsen NA, Al-Bassam J, Wei RR, Harrison SC (January 2007). "Structural analysis of Bub3 interactions in the mitotic spindle checkpoint". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 104 (4): 1201–6. Bibcode:2007PNAS..104.1201L. doi:10.1073/pnas.0610358104. PMC 1770893. PMID 17227844.
- Wang X, Babu JR, Harden JM, Jablonski SA, Gazi MH, Lingle WL, de Groen PC, Yen TJ, van Deursen JM (July 2001). "The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins". Biologik kimyo jurnali. 276 (28): 26559–67. doi:10.1074/jbc.M101083200. PMID 11352911.
- Kitagawa R, Rose AM (December 1999). "Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans". Tabiat hujayralari biologiyasi. 1 (8): 514–21. doi:10.1038/70309. PMID 10587648.
Tashqi havolalar
- Ted Salmon's lab: dividing cells movies. [1]
- Andrea Musacchio's lab: spindle checkpoint schemes. [2]
- http://www.uniprot.org/uniprot/O60566
