Apoptoz - Apoptosis
| Apoptoz | |
|---|---|
 An etopozid - davolangan DU145 prostata saratoni hujayrasi apoptotik jismlarning kaskadiga portlash. Sub-rasmlar 61 soatlik vaqtdan olingan vaqt o'tishi bilan mikroskopiya yordamida yaratilgan video miqdoriy fazali kontrastli mikroskop. Optik qalinligi rang bilan kodlangan. Borayotgan qalinlik bilan rang kul rangdan sariq, qizil, binafsha va nihoyat qora rangga o'zgaradi. Videoni "Hujayra: rasmlar kutubxonasi" da ko'ring | |
| Identifikatorlar | |
| MeSH | D017209 |
| Anatomik terminologiya | |

Apoptoz (dan.) Qadimgi yunoncha ςiς, apopsis, "tushib ketish") ning bir shakli dasturlashtirilgan hujayralar o'limi bu sodir bo'ladi ko'p hujayrali organizmlar.[1] Biokimyoviy hodisalar hujayralarning xarakterli o'zgarishiga olib keladi (morfologiya ) va o'lim. Ushbu o'zgarishlarga quyidagilar kiradi qon ketish, hujayralarning qisqarishi, yadro parchalanishi, xromatin kondensatsiyasi, xromosoma DNKning parchalanishi va global[noaniq ] mRNA yemirilish. Voyaga etgan odam o'rtacha 50 dan 70 gacha yo'qotadi milliard apoptoz tufayli har kuni hujayralar.[a] 8 yoshdan 14 yoshgacha bo'lgan o'rtacha odam bolasi uchun kuniga taxminan 20-30 milliard hujayralar nobud bo'ladi.[3]
Aksincha nekroz, bu o'tkir hujayraning shikastlanishidan kelib chiqadigan shikastlanadigan hujayralar o'limining shakli bo'lgan apoptoz organizmning hayot tsikli davomida afzalliklarni beradigan juda tartibga solinadigan va boshqariladigan jarayondir. Masalan, rivojlanayotgan odamda barmoqlar va oyoq barmoqlarini ajratish embrion raqamlar orasidagi hujayralar apoptozga uchraganligi sababli paydo bo'ladi. Nekrozdan farqli o'laroq, apoptoz hujayralar parchalarini hosil qiladi apoptotik jismlar bu fagotsit hujayralari hujayraning tarkibi atrofdagi hujayralarga tarqalib ketishi va ularga zarar etkazishidan oldin yutib yuborishi mumkin.[4]
Apoptoz boshlangandan keyin to'xtata olmasligi sababli, bu juda tartibga solingan jarayon. Apoptozni ikkita yo'ldan biri orqali boshlash mumkin. In ichki yo'l hujayra o'zini o'ldiradi, chunki u sezadi hujayralardagi stress, ichida tashqi yo'l hujayra boshqa hujayralar signallari tufayli o'zini o'ldiradi. Zaif tashqi signallar apoptozning ichki yo'lini ham faollashtirishi mumkin.[5] Ikkala yo'l ham faollashib hujayra o'limiga sabab bo'ladi kaspalar, qaysiki proteazlar, yoki oqsillarni parchalaydigan fermentlar. Ikkala yo'l ikkala tashabbuskor kaspazlarini faollashtiradi, so'ngra jallod kaspazlarni faollashtiradi, so'ngra hujayralarni oqsillarni ajratib tanazzulga olib o'ldiradi.
Biologik hodisa sifatida ahamiyatiga qo'shimcha ravishda nuqsonli apoptotik jarayonlar turli xil kasalliklarga aloqador. Haddan tashqari apoptoz sabablari atrofiya, ammo etarli miqdordagi hujayralar nazoratsiz ko'payishiga olib keladi, masalan saraton. Bunga o'xshash ba'zi omillar Fas retseptorlari va kaspazlar apoptozni rivojlantiradi, ba'zi a'zolari esa Bcl-2 oilasi oqsillar apoptozni inhibe qiladi.
Kashfiyot va etimologiya
Nemis olimi Karl Vogt birinchi bo'lib apoptoz tamoyilini 1842 yilda tasvirlab bergan. 1885 yilda anatomist Walther Flemming dasturlashtirilgan hujayralar o'limi jarayonini aniqroq tavsiflab berdi. Biroq, 1965 yilga qadargina bu mavzu qayta tiklandi. Elektron mikroskop yordamida to'qimalarni o'rganish paytida, Jon Fokson Ross Kerr Kvinslend universitetida apoptozni shikastlanadigan hujayralar o'limidan ajrata oldi.[6] Ushbu hodisani tavsiflovchi qog'oz nashr etilgandan so'ng, Kerr qo'shilishga taklif qilindi Alastair R. Currie, shu qatorda; shu bilan birga Endryu Uayli, Currining aspiranti bo'lgan,[7] Aberdin universitetida. 1972 yilda trio .da seminal maqola chop etildi Britaniya saraton jurnali.[8] Dastlab Kerr dasturlashtirilgan hujayra nekrozi atamasini ishlatgan edi, ammo maqolada hujayraning tabiiy o'lishi jarayoni deb nomlangan apoptoz. Kerr, Uayli va Kurri Yunon tili professori Jeyms Kormakka ishonishdi Aberdin universiteti, apoptoz atamasini taklif qilish bilan. Kerr qabul qildi Pol Ehrlich va Lyudvig Darmstaedter mukofoti apoptozni ta'rifi uchun 2000 yil 14 martda. U sovrinni Boston biologi bilan bo'lishdi H. Robert Xorvits.[9]
Ko'p yillar davomida na "apoptoz", na "dasturlashtirilgan hujayralar o'limi" juda keltirilgan atama emas edi. Ikki kashfiyot xujayralarni o'limini noaniqlikdan tadqiqotning asosiy sohasiga olib keldi: hujayraning o'limini boshqarish va effektor mexanizmlarining tarkibiy qismlarini aniqlash va hujayralar o'limidagi anormalliklarni inson kasalligi, xususan saraton kasalligi bilan bog'lash.
2002 yil Tibbiyot bo'yicha Nobel mukofoti bilan taqdirlandi Sidney Brenner, Horvits va Jon E. Sulston apoptozni boshqaruvchi genlarni aniqlaydigan ishlari uchun. Genlar nematodadagi tadqiqotlar natijasida aniqlandi C. elegans va bu genlarning gomologlari odamlarda apoptozni boshqarishda ishlaydi.

Yunon tilida apoptoz daraxtdan barglarning "tushishi" degan ma'noni anglatadi.[10] Yunon tili professori Kormak tibbiyotdan foydalanish atamasini qayta kiritdi, chunki bu ikki ming yil avval yunonlar uchun tibbiy ma'noga ega edi. Gippokrat bu atamani "suyaklarning qulashi" ma'nosida ishlatgan. Galen ma'nosini "qoraqo'tirlarni tomizish" ga qadar kengaytirdi. Kormak, ismni taklif qilganida, shubhasiz, bu ishlatilishidan xabardor edi. Munozara to'g'ri talaffuz bo'yicha davom etadi, fikr ikkinchi va talaffuz o'rtasida bo'linadi p jim (/æpəˈtoʊsɪs/ ap-ə-TOH-sis[11][12]) va ikkinchisi p talaffuz qilingan (/eɪpəpˈtoʊsɪs/),[11][13] asl yunon tilidagi kabi.[iqtibos kerak ] Ingliz tilida p yunoncha -pt- undosh klaster so'zning boshida odatda jim turadi (masalan.) pterodaktil, Ptolomey ), lekin unli tovush oldidagi shakllarni birlashtirishda ishlatilganda ifodalangan, xuddi vertolyot yoki hasharotlarning buyruqlari: diptera, lepidoptera, va boshqalar.
Asl Kerr, Uayli va Curri qog'ozida,[8] talaffuz bilan bog'liq izoh mavjud:
Biz ushbu atamani taklif qilgani uchun Aberdin universiteti yunon kafedrasi professori Jeyms Kormakdan juda minnatdormiz. "Apoptoz" so'zi (ςiς) yunon tilida gullardan yaproqchalarni yoki daraxtlardan barglarni "tushirish" yoki "tushish" ni ifodalash uchun ishlatiladi. Chiqarishni aniq ko'rsatish uchun biz ta'kidlashimiz kerakki, stress avvalgi hecada bo'lishi kerak, so'zning ikkinchi yarmi "ptosis" ("p" jim bilan), xuddi shu "tushish" ildizidan kelib chiqqan holda o'qiladi, va allaqachon yuqori ko'z qovog'ini tushishini tasvirlash uchun ishlatilgan.
Faollashtirish mexanizmlari


Apoptozning boshlanishi aktivizatsiya mexanizmlari bilan qat'iy tartibga solinadi, chunki apoptoz boshlangandan so'ng, u muqarrar ravishda hujayraning o'limiga olib keladi.[14][15] Ikkala eng yaxshi tushunilgan faollashtirish mexanizmi ichki yo'ldir (shuningdek, mitoxondrial tashqi yo'l) va tashqi yo'l.[16] The ichki yo'l hujayralar zo'riqishida hosil bo'lgan hujayra ichidagi signallar bilan faollashadi va mitoxondriyaning membranalararo bo'shligidan oqsillarni chiqarilishiga bog'liq.[17] The tashqi yo'l hujayra sirtidagi o'lim retseptorlari bilan bog'langan hujayradan tashqari ligandlar bilan faollashadi, bu esa hosil bo'lishiga olib keladi o'limga olib keladigan signalizatsiya majmuasi (DISC).[18]
Hujayra stressga javoban hujayra ichidagi apoptotik signalizatsiyani boshlaydi,[19] bu hujayralardagi o'z joniga qasd qilishga olib kelishi mumkin. Yadro retseptorlarining bog'lanishi glyukokortikoidlar,[20] issiqlik,[20] nurlanish,[20] ozuqa moddalarining etishmasligi,[20] virusli infektsiya,[20] gipoksiya,[20] erkin yog 'kislotalarining hujayra ichidagi konsentratsiyasining ortishi[21] va hujayra ichidagi o'sish kaltsiy diqqat,[22][23] masalan, membrananing shikastlanishi bilan, barchasi zararlangan hujayra tomonidan hujayra ichidagi apoptotik signallarning chiqishiga sabab bo'lishi mumkin. Kabi bir qator uyali komponentlar poli ADP riboz polimeraza, shuningdek, apoptozni tartibga solishda yordam berishi mumkin.[24] Yagona hujayraning dalgalanmaları stresli apoptozni eksperimental tadqiq qilishda kuzatilgan.[25][26]
Hujayra o'limining haqiqiy jarayoni fermentlar tomonidan cho'ktirilishidan oldin, apoptotik signallar tartibga soluvchi oqsillarning apoptoz yo'lini boshlashiga sabab bo'lishi kerak. Ushbu qadam, hujayraning o'lishi kerak bo'lmaganda, ushbu signallar hujayraning o'limiga olib keladi yoki jarayonni to'xtatishga imkon beradi. Bir nechta oqsillar ishtirok etadi, ammo tartibga solishning ikkita asosiy usuli aniqlandi: maqsadga yo'naltirish mitoxondriya funktsionallik,[27] yoki to'g'ridan-to'g'ri signalni uzatish adapter oqsillari apoptotik mexanizmlarga. Bir nechta toksinlarni o'rganish natijasida boshlangan tashqi yo'l hujayra ichidagi kaltsiy konsentratsiyasining o'sishi, bu giyohvand moddalar ta'siridan kelib chiqadi va bu kaltsiyni bog'laydigan proteaz orqali apoptozni keltirib chiqarishi mumkin. kalpain.
Ichki yo'l
Ichki yo'l mitoxondriyal yo'l deb ham ataladi. Mitoxondriya ko'p hujayrali hayot uchun juda muhimdir. Ularsiz hujayra to'xtaydi aerobik tarzda nafas oling va tezda o'ladi. Bu haqiqat ba'zi apoptotik yo'llar uchun asos bo'lib xizmat qiladi. Mitoxondriyani maqsad qilgan apoptotik oqsillar ularga har xil ta'sir qiladi. Ular membrana teshiklari hosil bo'lishi orqali mitoxondriyal shishishni keltirib chiqarishi yoki mitoxondriyal membrananing o'tkazuvchanligini oshirishi va apoptotik effektorlarning chiqib ketishiga olib kelishi mumkin.[20][28] Ular ichki yo'l bilan chambarchas bog'liq va o'smalar tashqi yo'lga qaraganda sezgirlik tufayli ichki yo'l orqali tez-tez paydo bo'ladi.[29] Buni ko'rsatadigan dalillarning tobora ko'payib borishi azot oksidi tarqalishiga yordam berish orqali apoptozni keltirib chiqarishi mumkin membrana potentsiali mitoxondriyadan kelib chiqadi va shuning uchun uni o'tkazuvchanroq qiladi.[30] Azot oksidi apoptozni faollashtiradigan keyingi yo'llarning signal molekulasi sifatida mumkin bo'lgan harakati orqali apoptozni boshlash va inhibe qilishda ishtirok etgan.[31]
Apoptoz paytida, sitoxrom v oqsillar harakati orqali mitoxondriyadan ajralib chiqadi Bax va Bak. Ushbu bo'shatish mexanizmi sirli, ammo tashqi membranaga kiritilgan Bax / Bakning ko'p sonli Bax / Bak homo va hetero-dimerlaridan kelib chiqadi.[32] Bir marta sitoxrom v ajralib chiqadi, u Apoptotik proteaz faollashtiruvchi omil bilan bog'lanadi - 1 (Apaf-1 ) va ATP, keyin bog'langan pro-kaspaz-9 sifatida tanilgan protein kompleksini yaratish apoptosoma. Apoptosoma pro-kaspazni faol shakliga yopishtiradi kaspaz-9, bu esa o'z navbatida effektorga pro-kaspazni ajratadi va faollashtiradi kaspaz-3.
Mitoxondriya shuningdek SMAC (ikkinchi mitoxondriyadan kelib chiqqan aktivator) deb nomlanuvchi oqsillarni chiqaradi kaspalar ) hujayraga sitozol mitoxondriya membranalarining o'tkazuvchanligi oshganidan keyin. SMAC ulanadi apoptozni inhibe qiluvchi oqsillar (IAP) bu bilan ularni o'chiradi va IAPlarning jarayonni hibsga olishiga yo'l qo'ymaydi va shuning uchun apoptozning davom etishiga imkon beradi. IAP shuningdek, odatda bir guruh faoliyatini bostiradi sistein proteazlari deb nomlangan kaspalar,[33] hujayraning degradatsiyasini amalga oshiradigan. Shuning uchun haqiqiy degradatsiyaga uchragan fermentlar bilvosita mitoxondriyal o'tkazuvchanlik bilan tartibga solinishi mumkin.
Tashqi yo'l
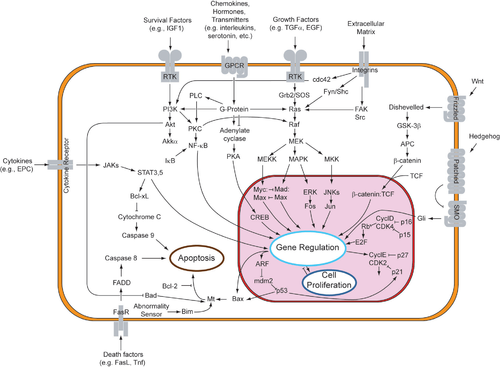


Sutemizuvchilardan apoptotik mexanizmlarni bevosita boshlashning ikkita nazariyasi taklif qilingan: TNF tomonidan induktsiya qilingan (o'simta nekrozi omil ) model va Fas-Fas ligand - vositachilik retseptorlari ishtirokidagi ikkala model TNF retseptorlari (TNFR) oilasi[34] tashqi signallarga qo'shilib.
TNF yo'li
TNF-alfa a sitokin asosan faollashtirilgan tomonidan ishlab chiqarilgan makrofaglar, va apoptozning asosiy tashqi vositachisidir. Inson tanasining aksariyat hujayralarida TNF-alfa uchun ikkita retseptor mavjud: TNFR1 va TNFR2. TNF-alfa ning TNFR1 bilan bog'lanishi oraliq membrana oqsillari TNF retseptorlari bilan bog'liq o'lim doirasi orqali kaspaz faollashuviga olib boradigan yo'lni boshlashi ko'rsatilgan (TRADD ) va Fas bilan bog'liq o'lim domeni oqsili (FADD ). cIAP1 / 2 ulanish orqali TNF-a signalizatsiyasini inhibe qilishi mumkin TRAF2. FLIP kaspaza-8 aktivatsiyasini inhibe qiladi.[35] Ushbu retseptorning bog'lanishi ham bilvosita faollashishiga olib kelishi mumkin transkripsiya omillari hujayra omon qolish va yallig'lanish reaktsiyalarida ishtirok etadi.[36] Shu bilan birga, TNFR1 orqali signal berish ham kaspazga bog'liq bo'lmagan holda apoptozni keltirib chiqarishi mumkin.[37] TNF-alfa va apoptoz o'rtasidagi bog'liqlik shuni ko'rsatadiki, nega TNF-alfa anormal ishlab chiqarilishi insonning bir qator kasalliklarida, ayniqsa, otoimmun kasalliklar. The TNF-alfa retseptorlari superfamily kabi o'lim retseptorlarini (DR) o'z ichiga oladi DR4 va DR5. Ushbu retseptorlar oqsil bilan bog'lanadiIz va vositachilik qiluvchi apoptoz. Apoptoz maqsadli saraton terapiyasining asosiy mexanizmlaridan biri ekanligi ma'lum.[38] Yaqinda TRAIL-ni taqlid qiladigan va saraton hujayralarida o'lim retseptorlari bilan bog'lanib, apoptozni keltirib chiqaradigan lyuminestsent iridiy kompleks-peptid duragaylari (IPH) yaratildi.[39]
- Fas yo'li
The fas retseptorlari (Birinchi apoptoz signal) - (shuningdek ma'lum Apo-1 yoki CD95) a transmembran oqsili bog'laydigan TNF oilasidan Fas ligand (FasL).[34] Fas va FasL o'rtasidagi o'zaro bog'liqlik hosil bo'lishiga olib keladi o'limga olib keladigan signalizatsiya majmuasi (DISC) tarkibiga FADD, kaspaz-8 va kaspaz-10 kiradi. Ba'zi turdagi hujayralarda (I tip) qayta ishlangan kaspaza-8 to'g'ridan-to'g'ri kaspaza oilasining boshqa a'zolarini faollashtiradi va hujayraning apoptozisining bajarilishini keltirib chiqaradi. Boshqa turdagi hujayralarda (II tip), Fas-DISC proapoptotik omillarni mitoxondriyadan chiqarilishini kuchayishiga va kaspaza-8ning kuchaytirilgan faollashuviga aylanadigan teskari aloqani boshlaydi.[40]
- Umumiy komponentlar
Keyingi TNF-R1 va Fas sutemizuvchilar hujayralarida aktivizatsiya[iqtibos kerak ] proapoptotik o'rtasidagi muvozanat (BAX,[41] BID, BAK, yoki YOMON ) va anti-apoptotik (Bcl-Xl va Bcl-2 ) a'zolari Bcl-2 oila barpo etildi. Ushbu muvozanat proapoptotikning nisbati homodimerlar mitoxondriyaning tashqi membranasida hosil bo'lgan Proapoptotik homodimerlardan sitoxrom c va SMAC kabi kaspaza aktivatorlarini chiqarish uchun mitoxondriyal membranani o'tkazuvchanligi talab qilinadi. Nopoptotik hujayralardagi normal hujayra sharoitida proapoptotik oqsillarni boshqarilishi to'liq tushunilmagan, ammo umuman olganda Bax yoki Bak faqat BH3 tarkibidagi oqsillarni faollashishi bilan faollashadi. Bcl-2 oila[iqtibos kerak ].
- Kaspalar
Kaspalar ER apoptotik signallarini uzatishda markaziy rol o'ynaydi. Kaspazlar - bu yuqori darajada konservalangan, sisteinga bog'liq bo'lgan aspartatga xos proteazlar. Kaspazlarning ikki turi mavjud: tashabbuskor kaspazlar, kaspaza 2,8,9,10,11,12 va effektor kaspazlar, kaspazlar 3,6,7. Boshlovchi kaspazlarning faollashishi o'ziga xos oligomerik bilan bog'lanishni talab qiladi faollashtiruvchi oqsil. Keyin effektor kaspazlari ushbu faol tashabbuskor kaspazlari orqali faollashadi proteolitik dekolte. Keyin faol effektor hujayralarni o'lishi dasturini amalga oshirish uchun hujayra ichidagi oqsillarni proteolitik ravishda parchalaydi.
- Kaspazdan mustaqil apoptotik yo'l
Shuningdek, AIF vositachiligida bo'lgan kaspaz-mustaqil apoptotik yo'l mavjud (apoptozni keltirib chiqaruvchi omil ).[42]
Amfibiyalarda apoptoz modeli
Amfibiya qurbaqasi Ksenopus laevis apoptoz mexanizmlarini o'rganish uchun ideal model tizim bo'lib xizmat qiladi. Aslida yod va tiroksin amfibiyalarda metamorfozdagi lichinka gillalari, dumlari va suyaklari hujayralarining ajoyib apoptozini rag'batlantiradi va ularning asab tizimi evolyutsiyasini rag'batlantiradi. qurbaqa.[43][44][45][46]
Apoptozning salbiy regulyatorlari
Apoptozning salbiy regulyatsiyasi hujayralar o'limining signalizatsiya yo'llarini inhibe qiladi, o'smalar hujayralar o'limidan qochib qutulishiga yordam beradi dorilarga qarshilik. Anti-apoptotik (Bcl-2) va pro-apoptotik (Bax) oqsillari o'rtasidagi nisbat hujayraning yashashi yoki o'lishini aniqlaydi.[47][48] Ko'pgina oqsillar oilalari antapoptotik omillarga bo'linadigan salbiy regulyator sifatida ishlaydi IAPlar va Bcl-2 kabi oqsillar yoki prosurvival omillar cFLIP, BNIP3, FADD, Akt va NF-DB.[49]
Proteolitik kaskad kaskad: hujayrani o'ldirish
Ko'p yo'llar va signallar apoptozga olib keladi, ammo ular hujayraning o'limiga olib keladigan yagona mexanizmga yaqinlashadi. Hujayra stimul olgandan so'ng, u faollashtirilgan proteolitik orqali hujayra organoidlarining uyushgan degradatsiyasiga uchraydi. kaspalar. Uyali organoidlarni yo'q qilishdan tashqari, mRNA hali to'liq tavsiflanmagan mexanizm tomonidan tez va global darajada buzilgan.[50] mRNA parchalanishi apoptozda juda erta boshlanadi.
Apoptozga uchragan hujayra bir qator xarakterli morfologik o'zgarishlarni ko'rsatadi. Dastlabki o'zgarishlarga quyidagilar kiradi:
- Retraktsiya tufayli hujayraning qisqarishi va yaxlitlanishi sodir bo'ladi lamellipodiya va oqsilli sitoskeletning kaspazlar bilan parchalanishi.[51]
- Sitoplazma zich bo'lib, organoidlar zich o'ralgan ko'rinadi.
- Xromatin kondensatsiyaga duchor bo'ladigan ixcham yamoqlarga aylanadi yadroviy konvert (shuningdek, perinuclear konvert sifatida ham tanilgan) sifatida tanilgan jarayonda piknoz, apoptozning o'ziga xos belgisi.[52][53]
- Yadro konvertlari uzilib qoladi va uning ichidagi DNK parchalanadi karorexeksiya. Yadro bir nechta diskretlarga bo'linadi xromatin tanalari yoki nukleosomal birliklar DNKning degradatsiyasi tufayli.[54]
Apoptoz tez o'sib boradi va uning mahsulotlari tezda yo'q qilinadi, bu esa klassik gistologiya bo'limlarini aniqlash yoki tasavvur qilishni qiyinlashtiradi. Karyoreksiya paytida, endonukleaza faollashishi DNKning qisqa parchalarini qoldiradi, ular muntazam ravishda o'lchamlari bilan ajralib turadi. Ular o'ziga xos "narvon" ko'rinishini beradi agar keyin jel elektroforez.[55] Uchun testlar DNK narvonlari apoptozni farqlash ishemik yoki toksik hujayralar o'limi.[56]
Apoptotik hujayralarni demontaj qilish

Apoptotik hujayrani yo'q qilishdan oldin, demontaj jarayoni mavjud. Apoptotik hujayralarni demontaj qilishda uchta tan olingan qadam mavjud:[58]
- Membranadan qon ketish: hujayra membranasida noma'lum tartibsiz kurtaklar paydo bo'ladi qon ketishi. Dastlab bu kichikroq sirt pufakchalari. Keyinchalik ular kattalashib, dinamik membrana qon tomirlariga aylanishi mumkin.[58] Apoptotik hujayra membranasi qonashining muhim regulyatori ROCK1 (rho bog'langan spiral o'z ichiga olgan protein kinaz 1).[59][60]
- Membrana o'simtalarining paydo bo'lishi: Ba'zi bir hujayralar, o'ziga xos sharoitlarda, membrana protrusionlari deb nomlangan hujayra membranasining turli uzun va ingichka kengayishlarini rivojlantirishi mumkin. Uch turi tasvirlangan: mikrotubula boshoq, apoptopodiya (o'lim oyoqlari) va munchoqli apoptopodiya (ikkinchisi munchoqlar qatorida ko'rinishga ega).[61][62][63] Pannexin 1 apoptopodiya va boncuklu apoptopodiya hosil bo'lishida ishtirok etadigan membrana kanallarining muhim tarkibiy qismidir.[62]
- Parchalanish: Hujayra ko'paytmaga bo'linadi pufakchalar deb nomlangan apoptotik jismlar, o'tadigan fagotsitoz. Plazma membranasining chiqib ketishi apoptotik jismlarni fagotsitlarga yaqinlashtirishga yordam beradi.
O'lik hujayralarni olib tashlash
O'lik hujayralarni qo'shni fagotsit hujayralari tomonidan olib tashlanishi deb nomlangan efferotsitoz.[64]Apoptozning so'nggi bosqichidan o'tgan o'lik hujayralar fagotsitotik molekulalarni namoyish etadi, masalan fosfatidilserin, ularning hujayralari yuzasida.[65] Fosfatidilserin odatda plazma membranasining ichki varaqasi yuzasida uchraydi, ammo apoptoz paytida hujayradan tashqari sirtga qayta taqsimlanadi scramblase.[66] Ushbu molekulalar hujayrani belgilaydi fagotsitoz makrofaglar kabi tegishli retseptorlarga ega hujayralar tomonidan.[67] Fagotsitlar tomonidan o'layotgan hujayralarni olib tashlash tartibsiz ravishda an paydo bo'lmaydi yallig'lanish reaktsiyasi.[68] Apoptoz paytida uyali RNK va DNK bir-biridan ajralib, turli xil apoptotik jismlarga ajratiladi; RNKni ajratish nukleolyar segregatsiya sifatida boshlanadi.[69]
Yo'lning nokautlari
Ko'pchilik nokautlar oqsillarning har birining funktsiyasini sinab ko'rish uchun apoptoz yo'llarida qilingan. Bunga qo'shimcha ravishda bir nechta kaspaslar APAF1 va FADD, yangi fenotipni aniqlash uchun mutatsiyaga uchragan. Shish nekrozi faktori (TNF) nokautini yaratish uchun gendan 3704-5364 nukleotidlarini o'z ichiga olgan ekzon chiqarildi. Ushbu ekzon hujayralardagi to'g'ri ishlov berish uchun zarur bo'lgan yuqori darajada saqlanib qolgan etuk TNF domenining bir qismini va etakchi ketma-ketligini kodlaydi. TNF - / - sichqonlar normal rivojlanadi va hech qanday qo'pol tuzilish yoki morfologik anomaliyalarga ega emas. Ammo, SRBC (qo'y eritrotsitlari) bilan immunizatsiya qilinganida, bu sichqonlar antikor javobining pishib etishmasligini ko'rsatdilar; ular normal darajada IgM hosil qila olishdi, ammo o'ziga xos IgG darajasini rivojlantira olmadilar. Apaf-1 - bu apoptozga olib keladigan kaspaz kaskadini boshlash uchun kaspaz 9 bilan parchalanish orqali aylanadigan oqsil. APAF-1 genidagi a - / - mutatsiya embrional o'limga olib kelishi sababli, APAF-1 - / - sichqonchani hosil qilish uchun gen tuzoq strategiyasidan foydalanilgan. Ushbu tahlil intragenik gen sintezini yaratish orqali gen funktsiyasini buzish uchun ishlatiladi. APAF-1 gen tuzog'ini hujayralarga kiritganda, ko'plab morfologik o'zgarishlar yuz beradi, masalan, umurtqa pog'onasi, interdigital to'ralarning davomiyligi va ochiq miya. Bundan tashqari, 12.5-embrion kundan keyin embrionlarning miyasi bir nechta tarkibiy o'zgarishlarni ko'rsatdi. APAF-1 hujayralari nurlanish kabi apoptoz stimullaridan himoyalangan. BAX-1 nokaut qilingan sichqon oldingi miyaning shakllanishini va ba'zi neyron populyatsiyalarda va o'murtqa shnurda hujayralardagi o'limning kamayishini namoyish etadi, bu esa motor neyronlarning ko'payishiga olib keladi.
Kaspaz oqsillari apoptoz yo'lining ajralmas qismidir, shuning uchun taqillatishlar turli xil zararli natijalarga olib keladi. Kaspaz 9 nokauti miyaning jiddiy rivojlanishiga olib keladi. Kaspaz 8 ta nokaut yurak etishmovchiligiga va shu bilan embrional o'limga olib keladi. Biroq, cre-lox texnologiyasidan foydalangan holda, periferik T hujayralarining ko'payishini, T hujayralarining reaktsiyasini buzilishini va asab naychasining yopilishida nuqsonni ko'rsatadigan kaspaz 8 nokauti yaratildi. Ushbu sichqonlar CD95, TNFR va boshqalar vositachiligidagi apoptozga chidamli, ammo ultrabinafsha nurlanish, ximioterapevtik dorilar va boshqa ogohlantirishlar natijasida paydo bo'lgan apoptozga chidamli emasligi aniqlandi. Va nihoyat, kaspaz 3 taqillatishi miyadagi ektopik hujayralar massasi va membrananing qon ketishi yoki yadro parchalanishi kabi g'ayritabiiy apoptotik xususiyatlar bilan ajralib turardi. Ushbu KO sichqonlarining ajoyib xususiyati shundaki, ular juda cheklangan fenotipga ega: Casp3, 9, APAF-1 KO sichqonlarida asab to'qimalarining deformatsiyalari mavjud va FADD va Casp 8 KO yurakning nuqsonli rivojlanishini ko'rsatdi, ammo KO ning ikkala turida ham boshqa organlar normal rivojlangan va ba'zi hujayralar turlari hali ham noma'lum proapoptotik yo'llar mavjudligini ko'rsatuvchi apoptotik stimulga sezgir edi.
Apoptotikni nekrotik (nekroptotik) hujayralardan farqlash usullari
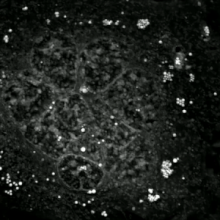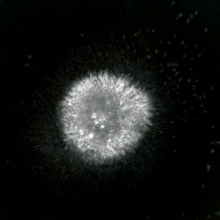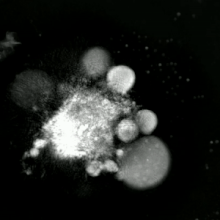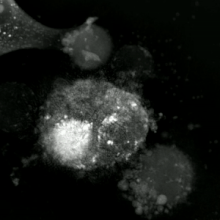
Apoptotik va nekrotik (nekroptotik) hujayralarni tahlil qilish uchun morfologiyani yorliqsiz tahlil qilish mumkin. jonli hujayralarni tasvirlash, vaqt o'tishi bilan mikroskopiya, oqim florotsitometriyasi va uzatish elektron mikroskopi. Shuningdek, hujayra yuzasi markerlarini tahlil qilish uchun turli xil biokimyoviy usullar mavjud (fosfatidilserinning ta'sirlanishi va hujayra o'tkazuvchanligi oqim sitometriyasi kabi uyali markerlar DNKning parchalanishi[70] (oqim sitometriyasi),[71] kaspazni faollashtirish, takliflarni ajratish va sitoxromni chiqarish (G'arbiy blotting ). Birlamchi va ikkilamchi nekrotik hujayralarni tahlil qilish yo'li bilan qanday ajratish mumkinligini bilish muhimdir superfant kaspazlar, HMGB1 va sitokeratin chiqarilishi uchun 18. Ammo nekrotik hujayraning o'limining aniq yuzasi yoki biokimyoviy belgilari hali aniqlanmagan va faqat salbiy belgilar mavjud. Bularga apoptotik markerlarning yo'qligi (kaspaza faollashuvi, sitoxrom s ning tarqalishi va oligonukleosomal DNKning parchalanishi) va hujayra o'lim markerlarining differentsial kinetikasi (fosfatidilserinning ta'sirlanishi va hujayra membranasining o'tkazuvchanligi) kiradi. Apoptozni nekroptotik hujayralardan ajratish uchun ishlatilishi mumkin bo'lgan metodlarni ushbu havolalarda topish mumkin.[72][73][74][75]
Kasallik



Nosoz yo'llar
Ko'p turli xil apoptotik yo'llar turli xil biokimyoviy tarkibiy qismlarni o'z ichiga oladi, ularning ko'plari hali tushunilmagan.[76] Yo'l tabiatda ozmi-ko'pmi ketma-ket bo'lgani uchun, bitta komponentni olib tashlash yoki o'zgartirish boshqasiga ta'sir ko'rsatishga olib keladi. Tirik organizmda bu halokatli ta'sirga ega bo'lishi mumkin, ko'pincha kasallik yoki buzuqlik shaklida. Har xil apoptotik yo'llarni o'zgartirish natijasida kelib chiqadigan har qanday kasallikni muhokama qilish maqsadga muvofiq emas, ammo ularning har biri ustida tushunchasi bir xil: yo'lning normal ishlashi buzilib, hujayraning o'tish qobiliyatini pasaytiradi. oddiy apoptoz. Natijada, "ishlatilish sanasi" dan o'tgan va har qanday nosoz uskunani ko'paytirib, nasl-nasabiga etkazadigan hujayra paydo bo'lib, hujayraning saraton yoki kasallikka chalinish ehtimolini oshiradi.
Ushbu kontseptsiyaning yaqinda tavsiflangan namunasini o'pka saratoni rivojlanishida ko'rish mumkin NCI-H460.[77] The Apoptoz oqsilining X bilan bog'langan inhibitori (XIAP ) haddan tashqari ta'sirlangan H460 hujayralarida hujayra chizig'i. XIAPlar kaspaza-9 ning qayta ishlangan shakli bilan bog'lanib, apoptotik aktivatorning faolligini bostiradi. sitoxrom v, shuning uchun haddan tashqari ekspression proapoptotik agonistlar miqdorining pasayishiga olib keladi. Natijada anti-apoptotik va proapoptotik effektorlarning muvozanati birinchisining foydasiga buziladi va zararlangan hujayralar o'lishga yo'naltirilganiga qaramay takrorlanishda davom etadi. Saraton hujayralarida apoptozni boshqarishda nuqsonlar ko'pincha transkripsiya omillarini boshqarish darajasida yuzaga keladi. Xususiy misol sifatida saraton kasalligidagi transkripsiya faktori NF-DB ni boshqaruvchi molekulalardagi nuqsonlar transkripsiya regulyatsiyasi rejimini va apoptotik signallarga ta'sirini o'zgartiradi, hujayra tegishli bo'lgan to'qimalarga bog'liqlikni kamaytiradi. Tashqi omon qolish signallaridan mustaqillikning bunday darajasi saraton metastazini keltirib chiqarishi mumkin.[78]
P53-ning regulyatsiyasi
O'simta-supressor oqsili p53 biokimyoviy omillar zanjiri tufayli DNK zararlanganda to'planadi. Ushbu yo'lning bir qismi alfa-interferon va beta-interferon, bu transkripsiyani keltirib chiqaradi p53 gen, natijada p53 oqsil darajasi oshadi va saraton hujayrasi-apoptoz kuchayadi.[79] p53 hujayraning takrorlanishini to'xtatib to'xtatadi hujayra aylanishi hujayraning tiklanishiga vaqt berish uchun G1 yoki interfazada, ammo zarar katta bo'lsa va tuzatish ishlari natija bermasa, bu apoptozni keltirib chiqaradi.[80] Tartibga solishning buzilishi p53 yoki interferon genlari apoptozning buzilishiga va shish paydo bo'lishiga olib keladi.
Inhibisyon
Apoptozni inhibe qilish natijasida ko'plab saraton, yallig'lanish kasalliklari va virusli infektsiyalar paydo bo'lishi mumkin. Dastlab hujayralar bilan bog'liq ravishda to'planish uyali proliferatsiyaning ko'payishi bilan bog'liq deb ishonilgan edi, ammo hozirda ma'lum bo'lishicha, bu hujayralar o'limining pasayishi bilan bog'liq. Ushbu kasalliklarning eng keng tarqalgani saraton kasalligi bo'lib, hujayralarning haddan tashqari ko'payishi kasalligi bo'lib, ko'pincha haddan tashqari ekspresiya bilan tavsiflanadi IAP oila a'zolari. Natijada, zararli hujayralar apoptoz induktsiyasiga g'ayritabiiy munosabatni boshdan kechirmoqda: tsiklni boshqaruvchi genlar (masalan, p53, ras yoki c-myc) kasal hujayralarda mutatsiyaga uchragan yoki faolsizlantirilgan va boshqa genlar (masalan, bcl-2) ham o'zgaradi. ularning shish paydo bo'lishi. Ba'zi apoptotik omillar mitoxondriyal nafas olish paytida juda muhimdir. sitoxrom S[81] Saraton hujayralarida apoptozning patologik inaktivatsiyasi glikolizga tez-tez uchraydigan nafas yo'llarining metabolik siljishi bilan bog'liq (kuzatuv "Warburg gipotezasi" deb nomlanadi).[82]
HeLa xujayrasi
Apoptoz HeLa[b] hujayralar hujayra tomonidan ishlab chiqarilgan oqsillar tomonidan inhibe qilinadi; bu inhibitor oqsillar retinoblastoma o'simtasini bostiruvchi oqsillarga qaratilgan.[83] Ushbu o'smani bostiruvchi oqsillar hujayra tsiklini tartibga soladi, ammo inhibitor oqsil bilan bog'langan holda faol bo'lmaydi.[83] HPV E6 va E7 - bu inson papillomavirusi bilan ifodalangan inhibitiv oqsillar, HPV esa HeLa hujayralari olinadigan serviks o'simtasining shakllanishiga javobgardir.[84] HPV E6 hujayralar aylanishini tartibga soluvchi p53 ning harakatsiz bo'lishiga olib keladi.[85] HPV E7 oqsillarni bostiruvchi retinoblastoma o'smasi bilan bog'lanadi va hujayra bo'linishini boshqarish qobiliyatini cheklaydi.[85] Ushbu ikkita inhibitor oqsillar apoptoz paydo bo'lishining oldini olish orqali HeLa hujayralarining o'lmasligi uchun qisman javobgardir.[86] CDV (Canine Distemper Virus) ushbu inhibitor oqsillarning mavjudligiga qaramay apoptozni keltirib chiqarishi mumkin. Bu muhim onkolitik CDV xususiyati: bu virus it lenfoma hujayralarini yo'q qilishga qodir. Onkoproteinlar E6 va E7 hanuzgacha p53 ni harakatsiz qoldiradilar, ammo ular virusli infeksiya stressidan kelib chiqqan kaspazlarning faollashuvidan qochib qutula olmaydilar. Ushbu onkolitik xususiyatlar CDV va lenfoma apoptozi o'rtasida istiqbolli aloqani ta'minladi, bu ikkala itni davolashning muqobil usullarini ishlab chiqishga olib kelishi mumkin limfoma va odamda Hodgkin bo'lmagan lenfoma. Hujayra siklidagi nuqsonlar ayrim o'simta hujayralarining kimyoviy terapiyasi yoki nurlanishiga chidamliligi uchun javobgar deb hisoblanadi, shuning uchun hujayra siklidagi nuqsonlarga qaramay apoptozni keltirib chiqaradigan virus saraton kasalligini davolash uchun foydalidir.[86]
Muolajalar
Signal bilan bog'liq kasalliklardan potentsial o'limni davolashning asosiy usuli kasallik apoptozning inhibisyoni yoki ortiqcha apoptoz tufayli kelib chiqqanligiga qarab, kasal hujayralardagi apoptozning sezgirligini oshirish yoki kamaytirishni o'z ichiga oladi. Masalan, muolajalar hujayra etishmovchiligidagi kasalliklarni davolash uchun apoptozni tiklashga va ortiqcha hujayralar o'limi bilan bog'liq kasalliklarni davolash uchun apoptotik chegarani oshirishga qaratilgan. Apoptozni rag'batlantirish uchun o'lim retseptorlari ligandlari sonini ko'paytirish mumkin (masalan, TNF yoki TRAIL), anti-apoptotik Bcl-2 yo'lini antagonize qilish yoki inhibitorni (IAP) inhibe qilish uchun Smac mimetikasini kiritish mumkin.[47] Herceptin, Iressa yoki Gleevec kabi agentlarning qo'shilishi hujayralarni velosipedda harakatlanishni to'xtatish uchun ishlaydi va o'sishni blokirovka qilish orqali apoptozning faollashuviga olib keladi va tirik qolish signalini keyingi oqimga yo'naltiradi. Nihoyat, p53- ni qo'shingMDM2 komplekslar p53 ni siqib chiqaradi va p53 yo'lini faollashtiradi, bu hujayra siklining to'xtashi va apoptozga olib keladi. O'lim signalizatsiyasi yo'li bo'ylab turli joylarda apoptozni rag'batlantirish yoki inhibe qilish uchun juda ko'p turli xil usullardan foydalanish mumkin.[87]
Apoptoz - bu organizmning har bir hujayrasiga xos bo'lgan ko'p bosqichli, ko'p bosqichli hujayra o'lim dasturi. Saraton kasalligida apoptoz hujayralarining bo'linish nisbati o'zgaradi. Saraton kasalligini ximioterapiya va nurlanish yordamida davolash, asosan, apoptozni qo'zg'atish orqali maqsad hujayralarni o'ldiradi.
Giperaktiv apoptoz
Boshqa tomondan, hujayralar o'limini nazorat qilishni yo'qotish (ortiqcha apoptozga olib keladi) neyrodejenerativ kasalliklarga, gematologik kasalliklarga va to'qimalarning shikastlanishiga olib kelishi mumkin. Shuni ta'kidlash kerakki, mitoxondriyal nafas olishga tayanadigan neyronlar Altsgeymer kabi neyrodejenerativ kasalliklarda apoptozga uchraydi.[88] va Parkinson.[89] ("teskari Warburg gipotezasi" deb nomlangan kuzatuv [90][81] ). Bundan tashqari, neyrodejenerativ kasalliklar va saraton o'rtasida teskari epidemiologik komorbidiya mavjud.[91] OIVning kuchayishi to'g'ridan-to'g'ri ortiqcha, tartibga solinmagan apoptoz bilan bog'liq. Sog'lom odamda CD4 + limfotsitlar soni suyak iligi hosil qilgan hujayralar bilan muvozanatda bo'ladi; ammo OIV bilan kasallangan bemorlarda bu muvozanat suyak iligi CD4 + hujayralarini qayta tiklay olmasligi tufayli yo'qoladi. OIV holatida, CD4 + limfotsitlari stimulyatsiya qilinganida, nazoratsiz apoptoz orqali tezlashib o'ladi, molekulyar darajada giperaktiv apoptoz Bcl-2 oilaviy oqsillarni boshqaruvchi signal yo'llarining nuqsonlaridan kelib chiqishi mumkin. BIM kabi apoptotik oqsillarning ko'payishi yoki ularning proteolizning pasayishi hujayralar o'limiga olib keladi va BIMning haddan tashqari faolligi sodir bo'lgan hujayralarga qarab bir qator patologiyalarni keltirib chiqarishi mumkin. Saraton xujayralari apoptozdan BIM ekspressionini bostiruvchi mexanizmlar yoki BIM proteolizining kuchayishi bilan qutulishi mumkin.[iqtibos kerak ]
Muolajalar
Muayyan kaspazlarni blokirovka qilish bo'yicha ishlarni inhibe qilishga qaratilgan muolajalar. Va nihoyat, Akt oqsil kinazasi hujayraning omon qolishiga ikki yo'l orqali yordam beradi. Akt fosforillanadi va Badni (Bcl-2 oilasi a'zosi) inhibe qiladi, bu esa Bad bilan o'zaro ta'sir qiladi 14-3-3 iskala, natijada Bcl dissotsiatsiyasi va shu bilan hujayralar omon qoladi. Akt shuningdek, IKKa-ni faollashtiradi, bu esa NF-kB aktivatsiyasiga va hujayralarning omon qolishiga olib keladi. Faol NF-kB Bcl-2 kabi anti-apoptotik genlarning ekspressionini keltirib chiqaradi, natijada apoptoz inhibisyonu paydo bo'ladi. NF-kB ishlatilgan stimullarga va hujayra turiga qarab antapoptotik rolni ham, proapoptotik rolni ham bajarishi aniqlandi.[92]
OIVning rivojlanishi
Ning rivojlanishi inson immunitet tanqisligi virusi ichiga yuqtirish OITS birinchi navbatda tükenmesi bilan bog'liq CD4 + T-yordamchi limfotsitlar tanadagi suyak iligi hujayralarni to'ldirish uchun juda tezkor tarzda, immunitetni buzilishiga olib keladi. T-yordamchi hujayralarni yo'q qilish mexanizmlaridan biri bu bir qator biokimyoviy yo'llardan kelib chiqadigan apoptozdir:[93]
- OIV fermentlari anti-apoptotikani o'chiradi Bcl-2. Bu to'g'ridan-to'g'ri hujayraning o'limiga olib kelmaydi, lekin tegishli signal olinishi kerak bo'lsa, apoptoz uchun hujayraning asosiy bosqichi. Bunga parallel ravishda, bu fermentlar proapoptotikni faollashtiradi procaspase-8, bu to'g'ridan-to'g'ri apoptozning mitoxondriyal hodisalarini faollashtiradi.
- OIV, Fas vositachiligidagi apoptozni keltirib chiqaradigan uyali oqsillarning darajasini oshirishi mumkin.
- OIV oqsillari miqdori kamayadi CD4 hujayra membranasida mavjud bo'lgan glikoprotein markeri.
- Hujayradan tashqari suyuqlikda mavjud bo'lgan bo'shatilgan virusli zarralar va oqsillar yaqin atrofdagi "yordamchi" T hujayralarida apoptozni keltirib chiqarishi mumkin.
- HIV decreases the production of molecules involved in marking the cell for apoptosis, giving the virus time to replicate and continue releasing apoptotic agents and virions into the surrounding tissue.
- The infected CD4+ cell may also receive the death signal from a cytotoxic T cell.
Cells may also die as direct consequences of viral infections. HIV-1 expression induces tubular cell G2/M arrest and apoptosis.[94] The progression from HIV to AIDS is not immediate or even necessarily rapid; HIV's cytotoxic activity toward CD4+ lymphocytes is classified as AIDS once a given patient's CD4+ cell count falls below 200.[95]
Researchers from Kumamoto University in Japan have developed a new method to eradicate HIV in viral reservoir cells, named "Lock-in and apoptosis." Using the synthesized compound Heptanoylphosphatidyl L-Inositol Pentakisphophate (or L-Hippo) to bind strongly to the HIV protein PR55Gag, they were able to suppress viral budding. By suppressing viral budding, the researchers were able to trap the HIV virus in the cell and allow for the cell to undergo apoptosis (natural cell death). Associate Professor Mikako Fujita has stated that the approach is not yet available to HIV patients because the research team has to conduct further research on combining the drug therapy that currently exists with this "Lock-in and apoptosis" approach to lead to complete recovery from HIV.[96]
Virusli infektsiya
Viral induction of apoptosis occurs when one or several cells of a living organism are infected with a virus, leading to cell death. Cell death in organisms is necessary for the normal development of cells and the cell cycle maturation.[97] It is also important in maintaining the regular functions and activities of cells.
Viruses can trigger apoptosis of infected cells via a range of mechanisms including:
- Qabul qiluvchilarni bog'lash
- Activation of oqsil kinazasi R (PKR)
- Interaction with p53
- Expression of viral proteins coupled to MHC proteins on the surface of the infected cell, allowing recognition by cells of the immune system (such as Tabiiy qotil va cytotoxic T cells ) that then induce the infected cell to undergo apoptosis.[98]
Itlarni yuqtiradigan virus (CDV) is known to cause apoptosis in central nervous system and lymphoid tissue of infected dogs in vivo and in vitro.[99]Apoptosis caused by CDV is typically induced via the tashqi yo'l faollashtiradigan caspases that disrupt cellular function and eventually leads to the cells death.[83] In normal cells, CDV activates caspase-8 first, which works as the initiator protein followed by the executioner protein caspase-3.[83] However, apoptosis induced by CDV in HeLa cells does not involve the initiator protein caspase-8. HeLa cell apoptosis caused by CDV follows a different mechanism than that in vero cell lines.[83] This change in the caspase cascade suggests CDV induces apoptosis via the intrinsic pathway, excluding the need for the initiator caspase-8. The executioner protein is instead activated by the internal stimuli caused by viral infection not a caspase cascade.[83]
The Oropouche virusi (OROV) is found in the family Bunyaviridae. The study of apoptosis brought on by Bunyaviridae was initiated in 1996, when it was observed that apoptosis was induced by the La Crosse virus into the kidney cells of baby hamsters and into the brains of baby mice.[100]
OROV is a disease that is transmitted between humans by the biting midge (Culicoides paraensis ).[101] U a deb nomlanadi zoonoz arbovirus and causes febrile illness, characterized by the onset of a sudden fever known as Oropouche fever.[102]
The Oropouche virus also causes disruption in cultured cells – cells that are cultivated in distinct and specific conditions. Bunga misolni ko'rish mumkin HeLa hujayralari, whereby the cells begin to degenerate shortly after they are infected.[100]
Dan foydalanish bilan gel elektroforezi, it can be observed that OROV causes DNK fragmentation in HeLa cells. It can be interpreted by counting, measuring, and analyzing the cells of the Sub/G1 cell population.[100] When HeLA cells are infected with OROV, the sitoxrom S is released from the membrane of the mitochondria, into the cytosol of the cells. This type of interaction shows that apoptosis is activated via an intrinsic pathway.[97]
In order for apoptosis to occur within OROV, viral uncoating, viral internalization, along with the replication of cells is necessary. Apoptosis in some viruses is activated by extracellular stimuli. However, studies have demonstrated that the OROV infection causes apoptosis to be activated through intracellular stimuli and involves the mitochondria.[100]
Many viruses encode proteins that can inhibit apoptosis.[103] Several viruses encode viral homologs of Bcl-2. These homologs can inhibit proapoptotic proteins such as BAX and BAK, which are essential for the activation of apoptosis. Examples of viral Bcl-2 proteins include the Epstein-Barr virusi BHRF1 protein and the adenovirus E1B 19K protein.[104] Some viruses express caspase inhibitors that inhibit caspase activity and an example is the CrmA protein of cowpox viruses. Whilst a number of viruses can block the effects of TNF and Fas. For example, the M-T2 protein of myxoma viruses can bind TNF preventing it from binding the TNF receptor and inducing a response.[105] Furthermore, many viruses express p53 inhibitors that can bind p53 and inhibit its transcriptional transactivation activity. As a consequence, p53 cannot induce apoptosis, since it cannot induce the expression of proapoptotic proteins. The adenovirus E1B-55K protein and the gepatit B virusi HBx protein are examples of viral proteins that can perform such a function.[106]
Viruses can remain intact from apoptosis in particular in the latter stages of infection. They can be exported in the apoptotik jismlar that pinch off from the surface of the dying cell, and the fact that they are engulfed by phagocytes prevents the initiation of a host response. This favours the spread of the virus.[105]
O'simliklar
Dasturlashtirilgan hujayralar o'limi in plants has a number of molecular similarities to that of animal apoptosis, but it also has differences, notable ones being the presence of a hujayra devori va yo'qligi immunitet tizimi o'lik hujayraning parchalarini olib tashlaydi. Immunitetga javoban, o'layotgan hujayra o'zini sindirish uchun moddalarni sintez qiladi va ularni a ga joylashtiradi vakuol hujayra o'lganda yorilib ketadi. Whether this whole process resembles animal apoptosis closely enough to warrant using the name apoptoz (as opposed to the more general dasturlashtirilgan hujayralar o'limi) aniq emas.[107][108]
Caspase-independent apoptosis
The characterization of the caspases allowed the development of caspase inhibitors, which can be used to determine whether a cellular process involves active caspases. Using these inhibitors it was discovered that cells can die while displaying a morphology similar to apoptosis without caspase activation.[109] Later studies linked this phenomenon to the release of AIF (apoptozni keltirib chiqaruvchi omil ) from the mitochondria and its translocation into the nucleus mediated by its NLS (nuclear localization signal). Inside the mitochondria, AIF is anchored to the inner membrane. In order to be released, the protein is cleaved by a calcium-dependent calpain protease.
Shuningdek qarang
Izohli izohlar
- ^ Note that the average human adult has more than 13 trillion cells (1.3×1013),[2] of which at most only 70 billion (7.0×1010) die per day. That is, about 5 out of every 1,000 cells (0.5%) die each day due to apoptosis.
- ^ HeLa cells are an immortalized cancer cell line used frequently in research. The cell line was established by removing cells directly from Henrietta etishmayapti, a cancer patient.
Iqtiboslar
- ^ Green D (2011). Means to an End: Apoptosis and other Cell Death Mechanisms. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-888-1.
- ^ Alberts, p. 2018-04-02 121 2.
- ^ Karam JA (2009). Apoptosis in Carcinogenesis and Chemotherapy. Niderlandiya: Springer. ISBN 978-1-4020-9597-9.
- ^ Alberts B, Jonson A, Lyuis J, Raff M, Roberts K, Valter P (2008). "Chapter 18 Apoptosis: Programmed Cell Death Eliminates Unwanted Cells". Hujayraning molekulyar biologiyasi (darslik) (5-nashr). Garland fani. p. 1115. ISBN 978-0-8153-4105-5.
- ^ Raychaudhuri S (August 2010). "A minimal model of signaling network elucidates cell-to-cell stochastic variability in apoptosis". PLOS ONE. 5 (8): e11930. arXiv:1009.2294. Bibcode:2010PLoSO...511930R. doi:10.1371/journal.pone.0011930. PMC 2920308. PMID 20711445.
- ^ Kerr JF (October 1965). "A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes". Patologiya va bakteriologiya jurnali. 90 (2): 419–35. doi:10.1002/path.1700900210. PMID 5849603.
- ^ Fan, texnologiya va tadqiqotlar agentligi. "Prof Andrew H. Wyllie – Lecture Abstract". Arxivlandi asl nusxasi on 2007-11-13. Olingan 2007-03-30.CS1 maint: mualliflar parametridan foydalanadi (havola)
- ^ a b Kerr JF, Uilli AH, Currie AR (1972 yil avgust). "Apoptoz: to'qima kinetikasida keng ta'sir ko'rsatadigan asosiy biologik hodisa". Britaniya saraton jurnali. 26 (4): 239–57. doi:10.1038 / bjc.1972.33. PMC 2008650. PMID 4561027.
- ^ O'Rourke MG, Ellem KA (2000). "John Kerr and apoptosis". Avstraliya tibbiyot jurnali. 173 (11–12): 616–17. doi:10.5694/j.1326-5377.2000.tb139362.x. PMID 11379508. S2CID 38265127.
- ^ Alberts, p. 1021.
- ^ a b Amerika merosi lug'ati Arxivlandi 2008 yil 30 iyun, soat Orqaga qaytish mashinasi
- ^ Apoptosis Interest Group (1999). "About apoptosis". Arxivlandi asl nusxasi 2006 yil 28 dekabrda. Olingan 2006-12-15.
- ^ "Definition of APOPTOSIS". www.webster.com. Arxivlandi asl nusxasi 2007-07-03 da. Olingan 2007-08-11.
- ^ Alberts, p. 1029.
- ^ Böhm I, Schild H (2003). "Apoptosis: the complex scenario for a silent cell death". Molekulyar tasvirlash va biologiya. 5 (1): 2–14. doi:10.1016/S1536-1632(03)00024-6. PMID 14499155.
- ^ Alberts, p. 1023.
- ^ Alberts, p. 1032.
- ^ Alberts, p. 1024.
- ^ Nirmala GJ va Lopus M (2020) Eukaryotlarda hujayra o'limining mexanizmlari. Hujayra Biol Toksikol, 36, 145-164. doi: /10.1007/s10565-019-09496-2. PMID 31820165
- ^ a b v d e f g Cotran RS, Kumar C (1998). Robbins Kasallikning patologik asoslari. Filadelfiya: W.B Saunders kompaniyasi. ISBN 978-0-7216-7335-6.
- ^ Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y (August 2003). "Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin". Biologik kimyo jurnali. 278 (34): 31861–70. doi:10.1074/jbc.m300190200. PMID 12805375.
- ^ Mattson MP, Chan SL (December 2003). "Calcium orchestrates apoptosis". Tabiat hujayralari biologiyasi. 5 (12): 1041–43. doi:10.1038/ncb1203-1041. PMID 14647298. S2CID 38427579.
- ^ Uğuz AC, Naziroğlu M, Espino J, Bejarano I, González D, Rodríguez AB, Pariente JA (December 2009). "Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and -9 activities". Membranalar biologiyasi jurnali. 232 (1–3): 15–23. doi:10.1007/s00232-009-9212-2. PMID 19898892. S2CID 22215706.
- ^ Chiarugi A, Moskowitz MA (July 2002). "Cell biology. PARP-1 – a perpetrator of apoptotic cell death?". Ilm-fan. 297 (5579): 200–01. doi:10.1126 / science.1074592. PMID 12114611. S2CID 82828773.
- ^ Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (March 2000). "The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant". Tabiat hujayralari biologiyasi. 2 (3): 156–62. doi:10.1038/35004029. PMID 10707086. S2CID 2283955.
- ^ Lee JK, Lu S, Madhukar A (October 2010). "Real-Time dynamics of Ca2+, caspase-3/7, and morphological changes in retinal ganglion cell apoptosis under elevated pressure". PLOS ONE. 5 (10): e13437. Bibcode:2010PLoSO...513437L. doi:10.1371/journal.pone.0013437. PMC 2956638. PMID 20976135.
- ^ Bejarano I, Espino J, González-Flores D, Casado JG, Redondo PC, Rosado JA, Barriga C, Pariente JA, Rodríguez AB (September 2009). "Role of Calcium Signals on Hydrogen Peroxide-Induced Apoptosis in Human Myeloid HL-60 Cells". International Journal of Biomedical Science. 5 (3): 246–56. PMC 3614781. PMID 23675144.
- ^ Gonzalez, D.; Bejarano, I .; Barriga, C.; Rodriguez, A.B.; Pariente, J.A. (2010). "Oxidative Stress-Induced Caspases are Regulated in Human Myeloid HL-60 Cells by Calcium Signal". Hozirgi signal uzatish terapiyasi. 5 (2): 181–186. doi:10.2174/157436210791112172.
- ^ Mohan S, Abdul AB, Abdelwahab SI, Al-Zubairi AS, Sukari MA, Abdullah R, Elhassan Taha MM, Ibrahim MY, Syam S (October 2010). "Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: its activation coupled with G0/G1 phase cell cycle arrest" (PDF). Etnofarmakologiya jurnali. 131 (3): 592–600. doi:10.1016/j.jep.2010.07.043. PMID 20673794. Arxivlandi asl nusxasi (PDF) 2019-04-26 da. Olingan 2019-07-05.
- ^ Brüne B (August 2003). "Nitric oxide: NO apoptosis or turning it ON?". Hujayra o'limi va differentsiatsiyasi. 10 (8): 864–69. doi:10.1038/sj.cdd.4401261. PMID 12867993.
- ^ Brüne B, von Knethen A, Sandau KB (October 1999). "Nitric oxide (NO): an effector of apoptosis". Hujayra o'limi va differentsiatsiyasi. 6 (10): 969–75. doi:10.1038/sj.cdd.4400582. PMID 10556974.
- ^ Uren RT, Iyer S, Kluck RM (August 2017). "Pore formation by dimeric Bak and Bax: an unusual pore?". London Qirollik Jamiyatining falsafiy operatsiyalari. B seriyasi, Biologiya fanlari. 372 (1726): 20160218. doi:10.1098/rstb.2016.0218. PMC 5483520. PMID 28630157.
- ^ Fesik SW, Shi Y (November 2001). "Structural biology. Controlling the caspases". Ilm-fan. 294 (5546): 1477–78. doi:10.1126/science.1062236. PMID 11711663. S2CID 11392850.
- ^ a b Wajant H (May 2002). "The Fas signaling pathway: more than a paradigm". Ilm-fan. 296 (5573): 1635–36. Bibcode:2002Sci...296.1635W. doi:10.1126/science.1071553. PMID 12040174. S2CID 29449108.
- ^ Chen G, Goeddel DV (May 2002). "TNF-R1 signaling: a beautiful pathway". Ilm-fan. 296 (5573): 1634–35. Bibcode:2002Sci...296.1634C. doi:10.1126/science.1071924. PMID 12040173. S2CID 25321662.
- ^ Goeddel, DV (2007). "Connection Map for Tumor Necrosis Factor Pathway". Science's STKE. 2007 (382): tw132. doi:10.1126/stke.3822007tw132. S2CID 85404086.
- ^ Chen W, Li N, Chen T, Han Y, Li C, Wang Y, He W, Zhang L, Wan T, Cao X (December 2005). "The lysosome-associated apoptosis-inducing protein containing the pleckstrin homology (PH) and FYVE domains (LAPF), representative of a novel family of PH and FYVE domain-containing proteins, induces caspase-independent apoptosis via the lysosomal-mitochondrial pathway". Biologik kimyo jurnali. 280 (49): 40985–95. doi:10.1074/jbc.M502190200. PMID 16188880.
- ^ Gerl R, Vaux DL (February 2005). "Apoptosis in the development and treatment of cancer". Kanserogenez. 26 (2): 263–70. doi:10.1093/carcin/bgh283. PMID 15375012.
- ^ Masum AA, Yokoi K, Hisamatsu Y, Naito K, Shashni B, Aoki S (September 2018). "Design and synthesis of a luminescent iridium complex-peptide hybrid (IPH) that detects cancer cells and induces their apoptosis". Bioorganik va tibbiy kimyo. 26 (17): 4804–16. doi:10.1016/j.bmc.2018.08.016. PMID 30177492.
- ^ Wajant H (2007). "Connection Map for Fas Signaling Pathway". Science's STKE. 2007 (380): tr1. doi:10.1126/stke.3802007tr1. S2CID 84909531.
- ^ Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB (January 2000). "Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells". Hujayra o'limi va differentsiatsiyasi. 7 (1): 102–11. doi:10.1038/sj.cdd.4400597. PMID 10713725.
- ^ Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (Fevral 1999) ). "Mitokondriyal apoptozni keltirib chiqaruvchi omilning molekulyar tavsifi". Tabiat. 397 (6718): 441–46. Bibcode:1999 yil Natur.397..441S. doi:10.1038/17135. PMID 9989411. S2CID 204991081.
- ^ Jewhurst K, Levin M, McLaughlin KA (2014). "Optogenetic Control of Apoptosis in Targeted Tissues of Xenopus laevis Embryos". Hujayra o'limi jurnali. 7: 25–31. doi:10.4137/JCD.S18368. PMC 4213186. PMID 25374461.
- ^ Venturi S (2011). "Yodning evolyutsion ahamiyati". Hozirgi kimyoviy biologiya. 5 (3): 155–62. doi:10.2174/187231311796765012.
- ^ Venturi, Sebastiano (2014). "Sog'liqni saqlash va kasallikdagi yod, PUFA va yodolipidlar: evolyutsion istiqbol". Inson evolyutsiyasi -. 29 (1–3): 185–205. ISSN 0393-9375.
- ^ Tamura K, Takayama S, Ishii T, Mavaribuchi S, Takamatsu N, Ito M (iyun 2015). "Apoptosis and differentiation of Xenopus tail-derived myoblasts by thyroid hormone". Molekulyar endokrinologiya jurnali. 54 (3): 185–92. doi:10.1530/JME-14-0327. PMID 25791374.
- ^ a b Jan R, Chaudhry G (2019). "Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics". Kengaytirilgan farmatsevtika byulleteni. 9 (2): 205–218. doi:10.15171/apb.2019.024. PMC 6664112. PMID 31380246.
- ^ Kale J, Osterlund EJ, Andrews DW (2018). "BCL-2 family proteins: changing partners in the dance towards death". Hujayra o'limi va farqlash. 25 (1): 65–80. doi:10.1038/cdd.2017.186. PMC 5729540. PMID 29149100.
- ^ Razaghi A, Heimann K, Schaeffer PM, Gibson SB (February 2018). "Negative regulators of cell death pathways in cancer: perspective on biomarkers and targeted therapies". Apoptoz. 23 (2): 93–112. doi:10.1007/s10495-018-1440-4. PMID 29322476. S2CID 3424489.
- ^ Thomas MP, Liu X, Whangbo J, McCrossan G, Sanborn KB, Basar E, Walch M, Lieberman J (May 2015). "Apoptosis Triggers Specific, Rapid, and Global mRNA Decay with 3' Uridylated Intermediates Degraded by DIS3L2". Hujayra hisobotlari. 11 (7): 1079–89. doi:10.1016/j.celrep.2015.04.026. PMC 4862650. PMID 25959823.
- ^ Böhm I (2003). "Disruption of the cytoskeleton after apoptosis induction by autoantibodies". Autoimmunitet. 36 (3): 183–89. doi:10.1080/0891693031000105617. PMID 12911286. S2CID 37887253.
- ^ Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, et al. (2000 yil avgust). "Two distinct pathways leading to nuclear apoptosis". Eksperimental tibbiyot jurnali. 192 (4): 571–80. doi:10.1084/jem.192.4.571. PMC 2193229. PMID 10952727.
- ^ Kihlmark M, Imreh G, Hallberg E (October 2001). "Sequential degradation of proteins from the nuclear envelope during apoptosis". Hujayra fanlari jurnali. 114 (Pt 20): 3643–53. PMID 11707516.
- ^ Nagata S (April 2000). "Apoptotic DNA fragmentation". Eksperimental hujayra tadqiqotlari. 256 (1): 12–8. doi:10.1006/excr.2000.4834. PMID 10739646.
- ^ Gong J, Traganos F, Darzynkiewicz Z (May 1994). "Gel elektroforezi va oqim sitometriyasi uchun qo'llaniladigan apoptotik hujayralardan DNK ekstraktsiyasini tanlash bo'yicha protsedura". Analitik biokimyo. 218 (2): 314–19. doi:10.1006 / abio.1994.1184. PMID 8074286.
- ^ Ivata M, Myerson D, Torok-Storb B, Zager RA (1994 yil dekabr). "Kislorod etishmovchiligi va oksidlovchi shikastlanishiga javoban buyrak naychali DNK narvonlarini baholash". Amerika nefrologiya jamiyati jurnali. 5 (6): 1307–13. PMID 7893995.
- ^ Smith A, Parkes MA, Atkin-Smith GK, Tixeira R, Poon IK (2017). "Cell disassembly during apoptosis". Tibbiyot bo'yicha WikiJournal. 4 (1). doi:10.15347/wjm/2017.008.
- ^ a b Tixeira R, Caruso S, Paone S, Baxter AA, Atkin-Smith GK, Hulett MD, Poon IK (March 2017). "Defining the morphologic features and products of cell disassembly during apoptosis". Apoptoz. 22 (3): 475–77. doi:10.1007/s10495-017-1345-7. PMID 28102458. S2CID 34648758.
- ^ Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (April 2001). "Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I". Tabiat hujayralari biologiyasi. 3 (4): 339–45. doi:10.1038/35070009. PMID 11283606. S2CID 2537726.
- ^ Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J (April 2001). "Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing". Tabiat hujayralari biologiyasi. 3 (4): 346–52. doi:10.1038/35070019. PMID 11283607. S2CID 36187702.
- ^ Moss DK, Betin VM, Malesinski SD, Lane JD (June 2006). "A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation". Hujayra fanlari jurnali. 119 (Pt 11): 2362–74. doi:10.1242/jcs.02959. PMC 1592606. PMID 16723742.
- ^ a b Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS (March 2014). "Unexpected link between an antibiotic, pannexin channels and apoptosis". Tabiat. 507 (7492): 329–34. Bibcode:2014Natur.507..329P. doi:10.1038/nature13147. PMC 4078991. PMID 24646995.
- ^ Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK (June 2015). "A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure". Tabiat aloqalari. 6: 7439. Bibcode:2015NatCo...6.7439A. doi:10.1038/ncomms8439. PMC 4490561. PMID 26074490.
- ^ Vandivier RW, Henson PM, Duglas IS (iyun 2006). "O'lganlarni dafn etish: apoptotik hujayralarni olib tashlashning (eferotsitoz) surunkali yallig'lanishli o'pka kasalligiga ta'siri". Ko'krak qafasi. 129 (6): 1673–82. doi:10.1378 / ko'krak.129.6.1673. PMID 16778289.
- ^ Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA (November 2003). "Phosphatidylserine receptor is required for clearance of apoptotic cells". Ilm-fan. 302 (5650): 1560–63. Bibcode:2003Sci...302.1560O. doi:10.1126/science.1087621. PMID 14645847. S2CID 36252352.
- ^ Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, et al. (2003 yil noyabr). "Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12". Ilm-fan. 302 (5650): 1563–66. Bibcode:2003Sci...302.1563W. doi:10.1126/science.1087641. PMID 14645848. S2CID 25672278.
- ^ Savill J, Gregory C, Haslett C (November 2003). "Cell biology. Eat me or die". Ilm-fan. 302 (5650): 1516–17. doi:10.1126/science.1092533. hdl:1842/448. PMID 14645835. S2CID 13402617.
- ^ Krysko DV, Vandenabeele P (2009-01-14). Phagocytosis of dying cells: from molecular mechanisms to human diseases. Springer. ISBN 978-1-4020-9292-3.
- ^ Halicka HD, Bedner E, Darzynkiewicz Z (November 2000). "Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis". Eksperimental hujayra tadqiqotlari. 260 (2): 248–56. doi:10.1006/excr.2000.5027. PMID 11035919.
- ^ Lozano GM, Bejarano I, Espino J, Gonsales D, Ortiz A, Garsiya JF, Rodrigez AB, Pariente JA (2009). "Zichlik gradiyenti sig'imi urug'lantirishni yaxshilash va bepusht erkaklarning DNKning parchalanishini kamaytirish uchun eng mos usuldir". Anadolu akusherlik va ginekologiya jurnali. 3 (1): 1–7.
- ^ Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F (January 1997). "Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis)". Sitometriya. 27 (1): 1–20. doi:10.1002/(sici)1097-0320(19970101)27:1<1::aid-cyto2>3.0.co;2-l. PMID 9000580.
- ^ Krysko DV, Vanden Berghe T, Parthoens E, D'Herde K, Vandenabeele P (2008). Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Enzimologiyadagi usullar. 442. pp. 307–41. doi:10.1016/S0076-6879(08)01416-X. ISBN 9780123743121. PMID 18662577.
- ^ Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P (March 2008). "Apoptosis and necrosis: detection, discrimination and phagocytosis". Usullari. 44 (3): 205–21. doi:10.1016/j.ymeth.2007.12.001. PMID 18314051.
- ^ Vanden Berghe T, Grootjans S, Goossens V, Dondelinger Y, Krysko DV, Takahashi N, Vandenabeele P (June 2013). "Determination of apoptotic and necrotic cell death in vitro and in vivo". Usullari. 61 (2): 117–29. doi:10.1016/j.ymeth.2013.02.011. PMID 23473780. Arxivlandi asl nusxasi 2019-11-05 da. Olingan 2019-11-05.
- ^ Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z (2011). "Apoptosis and beyond: cytometry in studies of programmed cell death". Recent Advances in Cytometry, Part B - Advances in Applications. Hujayra biologiyasidagi usullar. 103. pp. 55–98. doi:10.1016/B978-0-12-385493-3.00004-8. ISBN 9780123854933. PMC 3263828. PMID 21722800.
- ^ Thompson CB (March 1995). "Apoptosis in the pathogenesis and treatment of disease". Ilm-fan. 267 (5203): 1456–62. Bibcode:1995Sci...267.1456T. doi:10.1126/science.7878464. PMID 7878464. S2CID 12991980.
- ^ Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T, Oh-Hara T, Tsuruo T (February 2003). "Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide". Saraton kasalligini o'rganish. 63 (4): 831–37. PMID 12591734.
- ^ Vlahopoulos SA (avgust 2017). "NF-kB ning saraton kasalligini abberrant nazorati transkripsiya va fenotipik plastisitga, mezbon to'qimalarga bog'liqlikni kamaytirishga imkon beradi: molekulyar rejim". Saraton biologiyasi va tibbiyoti. 14 (3): 254–70. doi:10.20892 / j.issn.2095-3941.2017.0029. PMC 5570602. PMID 28884042.
- ^ Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. (July 2003). "Interferon-alfa / beta signalizatsiyasini p53 reaksiyalariga o'smani bostirish va antiviral himoya qilishda integratsiyasi". Tabiat. 424 (6948): 516–23. Bibcode:2003 yil Natur.424..516T. doi:10.1038 / nature01850. PMID 12872134.
- ^ Bernstein C, Bernstein H, Payne CM, Garewal H (June 2002). "DNKni tiklashning beshta asosiy yo'lidagi DNKni tiklash / apoptotik ikki tomonlama rolli oqsillar: kanserogenezdan xavfsiz himoya qilish". Mutatsion tadqiqotlar. 511 (2): 145–78. doi:10.1016/S1383-5742(02)00009-1. PMID 12052432.
- ^ a b Kaczanowski S (2016). "Apoptosis: its origin, history, maintenance and the medical implications for cancer and aging" (PDF). Fizik biol. 13 (3): 031001. Bibcode:2016PhBio..13c1001K. doi:10.1088/1478-3975/13/3/031001. PMID 27172135. Arxivlandi asl nusxasi (PDF) 2019-04-28 da. Olingan 2019-12-26.
- ^ Warburg O (1956 yil fevral). "Saraton hujayralarining kelib chiqishi to'g'risida". Ilm-fan. 123 (3191): 309–14. Bibcode:1956Sci ... 123..309W. doi:10.1126 / science.123.3191.309. PMID 13298683.
- ^ a b v d e f Del Puerto HL, Martins AS, Milsted A, Souza-Fagundes EM, Braz GF, Hissa B, Andrade LO, Alves F, Rajão DS, Leite RC, Vasconcelos AC (June 2011). "Canine distemper virus induces apoptosis in cervical tumor derived cell lines". Virusologiya jurnali. 8 (1): 334. doi:10.1186/1743-422X-8-334. PMC 3141686. PMID 21718481.
- ^ Liu HC, Chen GG, Vlantis AC, Tse GM, Chan AT, van Hasselt CA (March 2008). "Inhibition of apoptosis in human laryngeal cancer cells by E6 and E7 oncoproteins of human papillomavirus 16". Uyali biokimyo jurnali. 103 (4): 1125–43. doi:10.1002/jcb.21490. PMID 17668439. S2CID 1651475.
- ^ a b Niu XY, Peng ZL, Duan WQ, Wang H, Wang P (2006). "Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo". Xalqaro ginekologik saraton jurnali. 16 (2): 743–51. doi:10.1111/j.1525-1438.2006.00384.x. PMID 16681755.
- ^ a b Liu Y, McKalip A, Herman B (May 2000). "Human papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human keratinocytes to apoptosis induced by chemotherapeutic agents: roles of p53 and caspase activation". Uyali biokimyo jurnali. 78 (2): 334–49. doi:10.1002/(sici)1097-4644(20000801)78:2<334::aid-jcb15>3.3.co;2-6. PMID 10842327.
- ^ Boehm I (June 2006). "Apoptosis in physiological and pathological skin: implications for therapy". Hozirgi molekulyar tibbiyot. 6 (4): 375–94. doi:10.2174/156652406777435390. PMID 16900661.
- ^ LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G (January 1995). "The Alzheimer's A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice". Tabiat genetikasi. 9 (1): 21–30. doi:10.1038/ng0195-21. PMID 7704018. S2CID 20016461.
- ^ Mochizuki H, Goto K, Mori H, Mizuno Y (May 1996). "Histochemical detection of apoptosis in Parkinson's disease". Nevrologiya fanlari jurnali. 137 (2): 120–3. doi:10.1016/0022-510X(95)00336-Z. PMID 8782165. S2CID 44329454.
- ^ Demetrius LA, Magistretti PJ, Pellerin L (2014). "Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect". Fiziologiyadagi chegara. 5: 522. doi:10.3389/fphys.2014.00522. PMC 4294122. PMID 25642192.
- ^ Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, Palmer K, Russo A (July 2013). "Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study". Nevrologiya. 81 (4): 322–8. doi:10.1212/WNL.0b013e31829c5ec1. PMID 23843468. S2CID 22792702.
- ^ Farhana L, Dawson MI, Fontana JA (June 2005). "Apoptosis induction by a novel retinoid-related molecule requires nuclear factor-kappaB activation". Saraton kasalligini o'rganish. 65 (11): 4909–17. doi:10.1158/0008-5472.CAN-04-4124. PMID 15930313.
- ^ Alimonti JB, Ball TB, Fowke KR (July 2003). "Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS". Umumiy virusologiya jurnali. 84 (Pt 7): 1649–61. doi:10.1099/vir.0.19110-0. PMID 12810858.
- ^ Vashistha, Himanshu; Husain, Mohammad; Kumar, Dileep; Yadav, Anju; Arora, Shitij; Singhal, Pravin C. (2008). "HIV-1 Expression Induces Tubular Cell G2/M Arrest and Apoptosis". Buyrak etishmovchiligi. 30 (6): 655–664. doi:10.1080/08860220802134672. PMID 18661417.
- ^ Indiana universiteti sog'lig'i. "AIDS Defining Criteria | Riley". IU Health. Arxivlandi asl nusxasi on 2013-05-26. Olingan 2013-01-20.
- ^ Tateishi H, Monde K, Anraku K, Koga R, Hayashi Y, Ciftci HI, DeMirci H, Higashi T, Motoyama K, Arima H, Otsuka M, Fujita M (August 2017). "A clue to unprecedented strategy to HIV eradication: "Lock-in and apoptosis"". Ilmiy ma'ruzalar. 7 (1): 8957. Bibcode:2017NatSR...7.8957T. doi:10.1038/s41598-017-09129-w. PMC 5567282. PMID 28827668.
- ^ a b Indran IR, Tufo G, Pervaiz S, Brenner C (June 2011). "Recent advances in apoptosis, mitochondria and drug resistance in cancer cells". Biochimica et Biofhysica Acta (BBA) - Bioenergetika. 1807 (6): 735–45. doi:10.1016/j.bbabio.2011.03.010. PMID 21453675.
- ^ Everett H, McFadden G (April 1999). "Apoptosis: an innate immune response to virus infection". Mikrobiologiya tendentsiyalari. 7 (4): 160–65. doi:10.1016/S0966-842X(99)01487-0. PMID 10217831.
- ^ Nishi T, Tsukiyama-Kohara K, Togashi K, Kohriyama N, Kai C (November 2004). "Involvement of apoptosis in syncytial cell death induced by canine distemper virus". Qiyosiy immunologiya, mikrobiologiya va yuqumli kasalliklar. 27 (6): 445–55. doi:10.1016/j.cimid.2004.01.007. PMID 15325517.
- ^ a b v d Acrani GO, Gomes R, Proença-Módena JL, da Silva AF, Carminati PO, Silva ML, Santos RI, Arruda E (April 2010). "Apoptosis induced by Oropouche virus infection in HeLa cells is dependent on virus protein expression". Viruslarni o'rganish. 149 (1): 56–63. doi:10.1016/j.virusres.2009.12.013. PMID 20080135.
- ^ Azevedo RS, Nunes MR, Chiang JO, Bensabath G, Vasconcelos HB, Pinto AY, Martins LC, Monteiro HA, Rodrigues SG, Vasconcelos PF (June 2007). "Reemergence of Oropouche fever, northern Brazil". Rivojlanayotgan yuqumli kasalliklar. 13 (6): 912–15. doi:10.3201/eid1306.061114. PMC 2792853. PMID 17553235.
- ^ Santos RI, Rodrigues AH, Silva ML, Mortara RA, Rossi MA, Jamur MC, Oliver C, Arruda E (December 2008). "Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification". Viruslarni o'rganish. 138 (1–2): 139–43. doi:10.1016/j.virusres.2008.08.016. PMC 7114418. PMID 18840482.
- ^ Teodoro JG, Branton PE (March 1997). "Regulation of apoptosis by viral gene products". Virusologiya jurnali. 71 (3): 1739–46. doi:10.1128/jvi.71.3.1739-1746.1997. PMC 191242. PMID 9032302.
- ^ Polster BM, Pevsner J, Hardwick JM (March 2004). "Viral Bcl-2 homologs and their role in virus replication and associated diseases". Biochimica et Biofhysica Acta (BBA) - Molekulyar hujayralarni tadqiq qilish. 1644 (2–3): 211–27. doi:10.1016/j.bbamcr.2003.11.001. PMID 14996505.
- ^ a b Hay S, Kannourakis G (July 2002). "A time to kill: viral manipulation of the cell death program". Umumiy virusologiya jurnali. 83 (Pt 7): 1547–64. CiteSeerX 10.1.1.322.6923. doi:10.1099/0022-1317-83-7-1547. PMID 12075073.
- ^ Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Stürzbecher HW, Hoeijmakers JH, Harris CC (December 1995). "Abrogation of p53-induced apoptosis by the hepatitis B virus X gene". Saraton kasalligini o'rganish. 55 (24): 6012–16. PMID 8521383.
- ^ Collazo C, Chacón O, Borrás O (2006). "O'simliklardagi hujayralar dasturiy o'limi hayvonlarning apoptoziga o'xshaydi" (PDF). Biotexnología Aplicada. 23: 1-10. Arxivlandi asl nusxasi (PDF) 2009-03-03 da.
- ^ Dickman, Martin; Uilyams, Bret; Li, Yurong; De Figueiredo, Paul; Wolpert, Thomas (2017). "Reassessing apoptosis in plants". Tabiat o'simliklari. 3 (10): 773–779. doi:10.1038/s41477-017-0020-x. PMID 28947814. S2CID 3290201.
- ^ Xiang J, Chao DT, Korsmeyer SJ (December 1996). "BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 93 (25): 14559–63. Bibcode:1996PNAS...9314559X. doi:10.1073/pnas.93.25.14559. PMC 26172. PMID 8962091.
- ^ Kim, Jin Hee; Lee, C. H. (2009). "Atromentin-Induced Apoptosis in Human Leukemia U937 Cells". Mikrobiologiya va biotexnologiya jurnali. 19 (9): 946–950. doi:10.4014/jmb.0811.617. PMID 19809251. S2CID 11552839.
Umumiy bibliografiya
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). Hujayraning molekulyar biologiyasi (6-nashr). Garland fani. p. 2018-04-02 121 2. ISBN 978-0815344322.
Tashqi havolalar
- Apoptosis & cell surface[doimiy o'lik havola ]
- Apoptosis & Caspase 3, Proteoliz xaritasi - animatsiya
- Apoptosis & Caspase 8, Proteoliz xaritasi - animatsiya
- Apoptosis & Caspase 7, Proteoliz xaritasi - animatsiya
- Apoptosis MiniCOPE Dictionary – list of apoptosis terms and acronyms
- Apoptosis (Programmed Cell Death) – The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
- Apoptosis Research Portal
- Apoptosis Info Apoptosis protocols, articles, news, and recent publications.
- Database of proteins involved in apoptosis
- Apoptosis Video
- Apoptosis Video (WEHI on YouTube )
- The Mechanisms of Apoptosis Kimball's Biology Pages. Simple explanation of the mechanisms of apoptosis triggered by internal signals (bcl-2), along the caspase-9, caspase-3 and caspase-7 pathway; and by external signals (FAS and TNF), along the caspase 8 pathway. Kirish 25 mart 2007 yil.
- WikiPathways – Apoptosis pathway
- "Finding Cancer's Self-Destruct Button". CR magazine (Spring 2007). Article on apoptosis and cancer.
- Xiaodong Wang's lecture: Introduction to Apoptosis
- Robert Horvitz's Short Clip: Discovering Programmed Cell Death
- The Bcl-2 Database
- DeathBase: a database of proteins involved in cell death, curated by experts
- European Cell Death Organization
- Apoptosis signaling pathway created by Cusabio
