Rentgenologik kristallografiya - X-ray crystallography - Wikipedia

Rentgenologik kristallografiya (XRC) - a ning atom va molekulyar tuzilishini aniqlovchi eksperimental fan kristall, unda kristalli struktura voqea nurini keltirib chiqaradi X-nurlari ga diffraktsiya ko'plab aniq yo'nalishlarga. Ushbu difraksiyalangan nurlarning burchaklari va intensivligini o'lchab, a kristalograf zichligining uch o'lchovli rasmini chiqarishi mumkin elektronlar kristall ichida Bundan elektron zichligi, kristaldagi atomlarning o'rtacha pozitsiyalarini, shuningdek ularni aniqlash mumkin kimyoviy aloqalar, ularning kristallografik buzilish va boshqa har xil ma'lumotlar.
Chunki ko'plab materiallar kristallarni hosil qilishi mumkin - masalan tuzlar, metallar, minerallar, yarim o'tkazgichlar, shuningdek, turli noorganik, organik va biologik molekulalar - rentgen kristallografiyasi ko'plab ilmiy sohalarni rivojlantirishda muhim ahamiyatga ega. Dastlabki o'n yilliklarda ushbu usul atomlarning o'lchamlarini, kimyoviy bog'lanish uzunligi va turlarini va turli xil materiallar, xususan minerallar va qotishmalar. Usul ko'plab biologik molekulalarning, shu jumladan, tuzilishi va funktsiyalarini ham ochib berdi vitaminlar, giyohvand moddalar, oqsillar va nuklein kislotalar kabi DNK. X-nurli kristallografiya hanuzgacha yangi materiallarning atom tuzilishini tavsiflashda va boshqalarga o'xshash ko'rinadigan aniq materiallarda asosiy usul hisoblanadi. tajribalar. Rentgen kristalli tuzilmalar g'ayrioddiy narsalarni ham hisobga olishi mumkin elektron yoki elastik materialning xossalari, kimyoviy ta'sirlar va jarayonlarni yoritib beradi yoki asos bo'lib xizmat qiladi kasalliklarga qarshi dori vositalarini loyihalash.
Bir kristalli rentgen difraksiyasini o'lchashda kristall a ga o'rnatiladi goniometr. Goniometr kristalni tanlangan yo'nalishlarga joylashtirish uchun ishlatiladi. Kristal mayda fokuslangan holda yoritilgan monoxromatik rentgen nurlari nurlari, hosil qiluvchi a difraktsiya naqshlari sifatida tanilgan muntazam ravishda joylashgan joylarning aks ettirishlar. Turli yo'nalishlarda olingan ikki o'lchovli tasvirlar matematik usuli yordamida kristall ichidagi elektronlar zichligining uch o'lchovli modeliga aylantirildi. Furye o'zgarishi, namuna uchun ma'lum bo'lgan kimyoviy ma'lumotlar bilan birlashtirilgan. Kristallar juda kichik bo'lsa yoki ularning ichki tuzilishida etarlicha bir xil bo'lmasa, past aniqlik (loyqa) yoki hatto xatolarga olib kelishi mumkin.
X-nurli kristallografiya atom tuzilishini aniqlashning bir qancha boshqa usullari bilan bog'liq. Shunga o'xshash difraktsiya naqshlari elektronlarni tarqalishi yoki tomonidan ishlab chiqarilishi mumkin neytronlar, xuddi shunday talqin etiladi Furye transformatsiyasi. Agar etarlicha kattalikdagi bitta kristallarni olish imkoni bo'lmasa, unchalik batafsil bo'lmagan ma'lumotlarni olish uchun turli xil boshqa rentgen usullarini qo'llash mumkin; bunday usullarga quyidagilar kiradi tolaning difraksiyasi, chang difraksiyasi va (agar namuna kristallanmagan bo'lsa) kichik burchakli rentgen nurlari (SAXS) .Agar tekshirilayotgan materiallar faqat nanokristalli kukunlari yoki zaif kristallikdan aziyat chekadi, usullari elektron kristallografiyasi atom tuzilishini aniqlash uchun qo'llanishi mumkin.
Yuqorida aytib o'tilgan barcha rentgen diffraktsiya usullari uchun tarqalish elastik; tarqoq rentgen nurlari bir xil to'lqin uzunligi kiruvchi rentgen. Aksincha, elastik emas X-nurlarini sochish usullari namuna qo'zg'alishini o'rganishda foydalidir plazmonlar, kristalli va orbital hayajonlar, magnonlar va fononlar, uning atomlarining taqsimlanishidan ko'ra.[1]
Tarix
Kristallar va rentgen nurlarining dastlabki ilmiy tarixi

Kristallar uzoq vaqt muntazamligi va simmetriyasiga qoyil qolgan bo'lsalar-da, 17-asrgacha ilmiy tadqiq qilinmagan. Yoxannes Kepler uning ishida faraz qilingan Strena seu de Nive Sexangula Olti burchakli simmetriya (olti burchakli qorning yangi yil sovg'asi) (1611) qor parchalari kristallari sharsimon suv zarralarini muntazam ravishda qadoqlash bilan bog'liq edi.[2]

Daniyalik olim Nikolas Steno (1669) kristalli simmetriyani eksperimental tekshiruvlarini boshlagan. Steno ma'lum bir turdagi kristallarning har bir namunasida yuzlar orasidagi burchaklar bir xil ekanligini ko'rsatdi,[3] va Rene Just Hauy (1784) kristalning har bir yuzini bir xil shakldagi va o'lchamdagi bloklarni oddiy stakalash naqshlari bilan tavsiflash mumkinligini aniqladi. Shuning uchun, Uilyam Hallous Miller 1839 yilda har bir yuzga uchta kichik butun sonlardan iborat noyob yorliq berishga muvaffaq bo'ldi Miller indekslari bugungi kunda kristalli yuzlarni aniqlash uchun ishlatilayotgan. Hauyning tadqiqotlari kristallar muntazam uch o'lchovli massiv (a.) Degan to'g'ri fikrga olib keldi Bravais panjarasi ) atomlari va molekulalar; bitta birlik hujayrasi majburiy ravishda perpendikulyar bo'lmagan uchta asosiy yo'nalish bo'yicha cheksiz takrorlanadi. 19-asrda kristalning mumkin bo'lgan simmetriyalarining to'liq katalogi ishlab chiqilgan Yoxan Gessel,[4] Auguste Bravais,[5] Evgraf Fedorov,[6] Artur Shonflyus[7] va (kechikib) Uilyam Barlou (1894). Mavjud ma'lumotlar va fizik mulohazalardan Barlow 1880-yillarda rentgen kristallografiyasi bilan tasdiqlangan bir nechta kristalli tuzilmalarni taklif qildi;[8] ammo, mavjud ma'lumotlar 1880-yillarda uning modellarini yakuniy deb qabul qilish uchun juda kam edi.
Vilgelm Rentgen xuddi 1895 yilda xuddi kristall simmetriyasini o'rganish yakunlanayotgan paytda rentgen nurlarini kashf etdi. Fiziklar rentgen nurlarining tabiatiga shubha bilan qarashgan, ammo tez orada ularning to'lqinlari ekanligiga shubha qilishgan elektromagnit nurlanish, shakli yorug'lik. The Maksvell nazariyasi elektromagnit nurlanish olimlar orasida yaxshi qabul qilindi va tomonidan o'tkazilgan tajribalar Charlz Glover Barkla rentgen nurlari elektromagnit to'lqinlar, shu jumladan ko'ndalang bilan bog'liq hodisalarni namoyish etganligini ko'rsatdi qutblanish va spektral chiziqlar ko'rinadigan to'lqin uzunliklarida kuzatilganlarga o'xshash. Laboratoriyasida bitta bo'lak tajribalar Arnold Sommerfeld rentgen nurlari a to'lqin uzunligi taxminan 1 dan angstrom. X-nurlari nafaqat to'lqinlar, balki ular hamdir fotonlar, va zarracha xususiyatlariga ega. Albert Eynshteyn foton kontseptsiyasini 1905 yilda taqdim etdi,[9] ammo u 1922 yilgacha keng qabul qilinmadi,[10][11] qachon Artur Kompton uni rentgen nurlarining elektronlardan tarqalishi bilan tasdiqladi.[12] X-nurlarining zarrachalarga o'xshash xususiyatlari, masalan, ularning gazlarni ionlashtirishi turtki bergan edi Uilyam Genri Bragg 1907 yilda rentgen nurlari bo'lganligi haqida bahslashish emas elektromagnit nurlanish.[13][14][15][16] Braggning fikri ommalashmagan va kuzatuvlar isbotlangan Rentgen difraksiyasi tomonidan Maks fon Laue 1912 yilda[17] aksariyat olimlar uchun rentgen nurlari elektromagnit nurlanish shaklidir.
Rentgen difraksiyasi
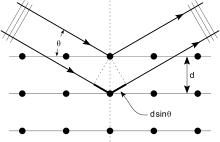
Kristallar atomlarning doimiy massivlari bo'lib, rentgen nurlarini elektromagnit nurlanish to'lqinlari deb hisoblash mumkin. Atomlar rentgen to'lqinlarini birinchi navbatda atomlarning elektronlari orqali tarqatadi. Dengiz chiroqiga urilgan okean to'lqini dengizdan chiqadigan ikkilamchi dumaloq to'lqinlarni hosil qilgani kabi, elektronga tushgan rentgen nurlari ham elektrondan chiqadigan ikkilamchi sferik to'lqinlarni hosil qiladi. Ushbu hodisa sifatida tanilgan elastik tarqalish, va elektron (yoki dengiz chiroqi) deb nomlanadi sochuvchi. Muntazam ravishda tarqaladigan massiv sharsimon to'lqinlarning muntazam massivini hosil qiladi. Garchi bu to'lqinlar bir-birini aksariyat yo'nalishlarda bekor qilsa ham halokatli aralashuv, ular tomonidan aniqlangan bir necha aniq yo'nalishlarda konstruktiv ravishda qo'shiladi Bragg qonuni:
Bu yerda d diffraktsion tekisliklar orasidagi bo'shliq, tushish burchagi, n har qanday butun son, va λ - nurning to'lqin uzunligi. Ushbu aniq yo'nalishlar dog'lar ko'rinishida ko'rinadi difraktsiya naqshlari deb nomlangan aks ettirishlar. Shunday qilib, rentgen diffraktsiyasi elektromagnit to'lqin (rentgen) natijasida tarqaluvchilarning muntazam massiviga (kristal ichidagi atomlarning takroriy joylashuvi) ta'sir qiladi.
Difraksion naqsh hosil qilish uchun rentgen nurlaridan foydalaniladi, chunki ularning to'lqin uzunligi g odatda kattalikning kattaligi (1-100 angstrom) oraliq bilan bir xil d kristaldagi tekisliklar orasida. Printsipial ravishda, tarqaluvchilarning doimiy qatoriga ta'sir qiladigan har qanday to'lqin hosil bo'ladi difraktsiya, oldindan taxmin qilinganidek Franchesko Mariya Grimaldi 1665 yilda. Difraksiyani sezilarli darajada oshirish uchun sochuvchilar orasidagi masofa va ta'sir ko'rsatayotgan to'lqinning to'lqin uzunligi kattaligiga o'xshash bo'lishi kerak. Masalan, quyosh nuri parranda tuklari orqali diffraktsiyasi haqida xabar berilgan Jeyms Gregori keyingi 17-asrda. Birinchi sun'iy difraksion panjaralar chunki ko'rinadigan yorug'lik tomonidan qurilgan Devid Rittenxaus 1787 yilda va Jozef fon Fraunhofer 1821 yilda. Ammo ko'rinadigan yorug'lik juda uzun to'lqin uzunligiga ega (odatda 5500 angstrom), kristallarning difraksiyasini kuzatish uchun. Birinchi rentgen difraksiyasi tajribalaridan oldin, kristaldagi panjara tekisliklari orasidagi masofalar aniq ma'lum emas edi.
Kristallardan a sifatida foydalanish mumkin degan fikr difraksion panjara uchun X-nurlari o'rtasidagi suhbatda 1912 yilda paydo bo'lgan Pol Piter Evald va Maks fon Laue ichida Ingliz bog'i yilda Myunxen. Evald tezis uchun kristallarning rezonator modelini taklif qilgan edi, ammo bu model yordamida tasdiqlanib bo'lmadi ko'rinadigan yorug'lik, chunki to'lqin uzunligi rezonatorlar orasidagi masofadan ancha katta edi. Von Lau bunday kichik oraliqlarni kuzatish uchun to'lqin uzunligining qisqaroq elektromagnit nurlanishi kerakligini anglab etdi va rentgen nurlari kristallardagi birlik-hujayra oralig'i bilan taqqoslanadigan to'lqin uzunligiga ega bo'lishi mumkinligini taxmin qildi. Von Laue ikkita texnik xodim - Valter Fridrix va uning yordamchisi Pol Kniping bilan birgalikda rentgen nurlari nurlarini nurlantirish uchun ishlagan. mis sulfat kristallini oling va uning difraksiyasini a ga yozing fotografiya plitasi. Ishlab chiqilgandan so'ng, plastinka markaziy nur tomonidan ishlab chiqarilgan nuqta atrofida kesishgan doiralar shaklida joylashtirilgan juda ko'p aniqlangan dog'larni ko'rsatdi.[17][18] Fon Laue kristaldagi sochilish burchaklari va birlik-xujayralar oralig'ining o'lchamlari va yo'nalishini bir-biriga bog'laydigan qonunni ishlab chiqdi, u uchun unga Fizika bo'yicha Nobel mukofoti 1914 yilda.[19]
Tarqoqlik
Da tasvirlanganidek Quyidagi matematik lotin, rentgen nurlarining tarqalishi kristall ichidagi elektronlar zichligi bilan aniqlanadi. X-nurining energiyasi valent elektronga qaraganda ancha katta bo'lgani uchun, tarqalish quyidagicha modellashtirilishi mumkin. Tomson sochilib ketmoqda, elektromagnit nurning erkin elektron bilan o'zaro ta'siri. Ushbu model odatda tarqalgan nurlanishning qutblanishini tavsiflash uchun qabul qilingan.
Intensivligi Tomson sochilib ketmoqda massasi bo'lgan bitta zarracha uchun m va oddiy zaryad q bu:[20]
Demak, elektronga qaraganda ancha og'ir bo'lgan atom yadrolari tarqoq rentgen nuriga beparvo hissa qo'shadi.
1912 yildan 1920 yilgacha rivojlanish

Von Laening kashshof tadqiqotlaridan so'ng, bu soha tez rivojlandi, ayniqsa fiziklar Uilyam Lourens Bragg va uning otasi Uilyam Genri Bragg. 1912–1913 yillarda kichikroq Bragg rivojlandi Bragg qonuni, bu kuzatilgan tarqalishni kristal ichidagi bir tekis joylashgan tekisliklarning aksi bilan bog'laydi.[21][22][23] Braggs, otasi va o'g'li, kristallografiyada qilgan ishlari uchun fizika bo'yicha 1915 yilgi Nobel mukofotini bo'lishdi. Dastlabki tuzilmalar odatda sodda va bir o'lchovli simmetriya bilan ajralib turardi. Ammo keyingi o'n yilliklarda hisoblash va eksperimental usullar takomillashib borganligi sababli, birlik-katakchadagi atomlarning murakkab ikki va uch o'lchovli joylashuvi uchun ishonchli atom pozitsiyalarini chiqarish maqsadga muvofiq bo'ldi.
Molekula va minerallarning tuzilishini aniqlash uchun rentgen kristallografiyasining potentsiali - keyinchalik kimyoviy va gidrodinamik tajribalardan noaniq ma'lum bo'lgan - darhol amalga oshirildi. Dastlabki tuzilmalar oddiy noorganik kristallar va minerallar bo'lgan, ammo bular ham fizika va kimyoning asosiy qonunlarini ochib bergan. 1914 yilda "hal qilingan" (ya'ni aniqlangan) birinchi atom-rezolyutsiya tuzilishi shu edi osh tuzi.[24][25][26] Elektronlarning osh tuzi tarkibidagi taqsimoti shuni ko'rsatdiki, kristallar albatta tuzilishi shart emas kovalent bog'langan molekulalari va mavjudligini isbotladi ionli birikmalar.[27] Ning tuzilishi olmos o'sha yili hal qilindi,[28][29] uning kimyoviy bog'lanishlarining tetraedral joylashishini isbotlagan va C-C yagona bog'lanishining uzunligi 1,52 angstrom bo'lganligini ko'rsatgan. Boshqa dastlabki tuzilmalar kiritilgan mis,[30] kaltsiy ftoridi (CaF2, shuningdek, nomi bilan tanilgan florit), kaltsit (CaCO3) va pirit (FeS2)[31] 1914 yilda; shpinel (MgAl2O4) 1915 yilda;[32][33] The rutil va anataza shakllari titanium dioksid (TiO2) 1916 yilda;[34] pirokroit Mn (OH)2 va kengaytma bilan brusit Mg (OH)2 1919 yilda.[35][36] Shuningdek, 1919 yilda, natriy nitrat (NaNO3) va sezyum dikloroiodid (CsICl2) tomonidan aniqlandi Ralf Uolter Greystoun Vaykoff, va vursit (olti burchakli ZnS) tuzilishi 1920 yilda ma'lum bo'ldi.[37]
Ning tuzilishi grafit 1916 yilda hal qilingan[38] tegishli usul bilan chang difraksiyasi,[39] tomonidan ishlab chiqilgan Piter Debye va Pol Sherrer va mustaqil ravishda, tomonidan Albert Xall 1917 yilda.[40] Grafitning tuzilishi 1924 yilda bitta kristalli difraksiyadan ikki guruh mustaqil ravishda aniqlandi.[41][42] Xull shuningdek temir kabi turli metallarning tuzilishini aniqlash uchun kukun usulini qo'llagan[43] va magniy.[44]
Madaniy va estetik ahamiyatga ega
1951 yilda Festival Pattern Group Britaniya festivali to'qimachilik ishlab chiqaruvchilari va tajribali kristallograflarning rentgen kristallografiyasi asosida dantel va bosmalarni loyihalashtirish bo'yicha hamkorlikdagi guruhiga mezbonlik qildi. insulin, chinni gil va gemoglobin. Loyihaning etakchi olimlaridan biri Dr. Xelen Megav (1907-2002), o'sha paytda Kembrijdagi Kavndish laboratoriyasida tadqiqot ishlari bo'yicha direktor yordamchisi. Megaw kristalli diagrammalardan ilhom olgan va ularning dizayndagi imkoniyatlarini ko'rgan markaziy shaxslardan biri sifatida tan olingan.[45] 2008 yilda Londondagi Wellcome Collection "Pattern Festival" guruhida "Atomdan naqshgacha" deb nomlangan ko'rgazmani o'tkazdi.[45]
Kimyo va materialshunoslikka qo'shgan hissalari
X-nurli kristallografiya yaxshi tushunishga olib keldi kimyoviy aloqalar va kovalent bo'lmagan o'zaro ta'sirlar. Dastlabki tadqiqotlar natijasida atomlarning odatdagi radiusi aniqlandi va kimyoviy bog'lanishning ko'plab nazariy modellari tasdiqlandi, masalan olmos tarkibidagi uglerodning tetraedral bog'lanishi,[28] ammoniy geksaxloroplatinat (IV) da kuzatilgan metallarning oktahedral bog'lanishi,[46] va planar karbonat guruhida kuzatilgan rezonans[31] va aromatik molekulalarda.[47] Ketlin Lonsdeyl 1928 yil tuzilishi geksametilbenzol[48] ning olti burchakli simmetriyasini o'rnatdi benzol va alifatik C-C bog'lanishlari va aromatik C-C bog'lanishlari orasidagi bog'lanish uzunligining aniq farqini ko'rsatdi; bu topilma g'oyaga olib keldi rezonans kimyo taraqqiyoti uchun chuqur oqibatlarga olib kelgan kimyoviy aloqalar o'rtasida.[49] Uning xulosalari kutilgan edi Uilyam Genri Bragg, kimning modellarini nashr etgan naftalin va antrasen 1921 yilda boshqa molekulalarga asoslanib, erta shakli molekulyar almashtirish.[47][50]
1920-yillarda, Viktor Morits Goldschmidt va keyinroq Linus Poling kimyoviy jihatdan mumkin bo'lmagan tuzilmalarni yo'q qilish va atomlarning nisbiy o'lchamlarini aniqlash qoidalarini ishlab chiqdi. Ushbu qoidalar tuzilishiga olib keldi brookit (1928) va ning nisbiy barqarorligini tushunish rutil, brookit va anataza shakllari titanium dioksid.
Ikki bog'langan atom orasidagi masofa bog'lanish kuchliligi va uning sezgir o'lchovidir obligatsiya buyurtmasi; Shunday qilib, rentgen-kristallografik tadqiqotlar yanada ulanishning ekzotik turlarini kashf etishga olib keldi noorganik kimyo, masalan, metall-metall er-xotin bog'lanishlar,[51][52][53] metall-metall to'rtburchak aloqalar,[54][55][56] va uch markazli, ikki elektronli bog'lanishlar.[57] X-ray kristallografiyasi - yoki aniq aytganda, noelastik Kompton tarqalishi eksperiment - shuningdek, qisman kovalent xarakterga ega ekanligini isbotladi vodorod aloqalari.[58] Sohasida organometalik kimyo, ning rentgen tuzilishi ferrosen boshlagan ilmiy tadqiqotlar sendvich aralashmalari,[59][60] shu bilan birga Zayzaning tuzi "orqa bog'lash" va metall-pi komplekslari bo'yicha tadqiqotlarni rag'batlantirdi.[61][62][63][64] Va nihoyat, rentgen-kristalografiya rivojlanishida kashshof rol o'ynadi supramolekulyar kimyo, ayniqsa tuzilmalarini aniqlashtirishda toj efirlari va tamoyillari mezbon - mehmonlar kimyosi.
Rentgen difraksiyasi juda kuchli vosita katalizator rivojlanish. Ex-situ o'lchovlari materiallarning kristalli tuzilishini tekshirish yoki yangi konstruksiyalarni ochish uchun muntazam ravishda amalga oshiriladi. In-situ tajribalar reaksiya sharoitida katalizatorlarning strukturaviy barqarorligi to'g'risida har tomonlama tushuncha beradi.[65][66][67]
Moddiy fanlarda juda ko'p murakkab noorganik va organometalik kabi kristalli usullar yordamida tizimlar tahlil qilindi fullerenlar, metalloporfirinlar va boshqa murakkab birikmalar. Yagona kristalli difraktsiya ham farmatsevtika sanoati, bilan bog'liq so'nggi muammolar tufayli polimorflar. Bir kristalli konstruktsiyalarning sifatiga ta'sir qiluvchi asosiy omillar kristalning kattaligi va muntazamligi; qayta kristallanish kichik molekulali kristallarda ushbu omillarni yaxshilash uchun tez-tez ishlatiladigan usul. The Kembrijning tarkibiy ma'lumotlar bazasi 2019 yil iyun holatiga ko'ra 1 000 000 dan ortiq inshootlarni o'z ichiga oladi; ushbu tuzilmalarning 99% dan ortig'i rentgen difraksiyasi bilan aniqlandi.
Mineralogiya va metallurgiya

1920-yillardan boshlab rentgen diffraktsiyasi minerallarda atomlarning joylashishini aniqlashning asosiy usuli hisoblanadi metallar. X-ray kristallografiyasining qo'llanilishi mineralogiya tuzilishi bilan boshlandi granat 1924 yilda Menzer tomonidan aniqlangan. Tizimli rentgen-kristallografik tadqiqoti silikatlar 20-asrning 20-yillarida amalga oshirildi. Ushbu tadqiqot shuni ko'rsatdiki, Si /O nisbati o'zgaradi, silikat kristallari atom tuzilishida sezilarli o'zgarishlarni ko'rsatadi. Machatschki ushbu tushunchalarni foydali qazilmalar haqida kengaytirdi alyuminiy o'rnini bosuvchi kremniy silikatlar atomlari. X-ray kristallografiyasining birinchi qo'llanilishi metallurgiya xuddi shu tarzda 1920-yillarning o'rtalarida sodir bo'lgan.[69][70][71][72][73][74] Eng muhimi, Linus Poling qotishma Mg ning tuzilishi2Sn[75] uning murakkab ion kristallarining barqarorligi va tuzilishi haqidagi nazariyasiga olib keldi.[76]
2012 yil 17 oktyabrda Qiziqish uchun mo'ljallangan rover ustida Mars sayyorasi da "Roknest "ning birinchi rentgen difraksiyasi tahlilini o'tkazdi Mars tuprog'i. Rover-ning natijalari CheMin analizatori bir qancha minerallar mavjudligini, shu jumladan dala shpati, piroksenlar va olivin, va namunadagi Mars tuprog'i "ob-havo sharoitiga o'xshash" deb taxmin qildi bazalt tuproqlari "ning Gavayi vulqonlari.[68]
Dastlabki organik va kichik biologik molekulalar

Organik birikmaning birinchi tuzilishi, geksametilenetetramin, 1923 yilda hal qilindi.[77] Buning ortidan uzun zanjirli bir necha tadqiqotlar o'tkazildi yog 'kislotalari, ning muhim tarkibiy qismi bo'lgan biologik membranalar.[78][79][80][81][82][83][84][85][86] 1930-yillarda ikki o'lchovli murakkablikka ega bo'lgan ancha katta molekulalarning tuzilmalari hal etila boshlandi. Ning tuzilishi muhim avans edi ftalosiyanin,[87] bilan chambarchas bog'liq bo'lgan katta planar molekula porfirin molekulalari kabi biologiyada muhim ahamiyatga ega heme, korin va xlorofill.
Biologik molekulalarning rentgen-kristalografiyasi olib borildi Doro Crowfoot Hodkin, tuzilmalarini kim hal qildi xolesterin (1937), penitsillin (1946) va B vitamini12 (1956), unga mukofot berilgan Kimyo bo'yicha Nobel mukofoti 1964 yilda. 1969 yilda u tuzilishini hal qilishga muvaffaq bo'ldi insulin, u o'ttiz yildan ortiq ishlagan.[88]
Biologik makromolekulyar kristallografiya

Oqsillarning kristalli tuzilmalari (ular tartibsiz va xolesteroldan yuzlab marta kattaroq) tuzilishi bilan boshlanib, 1950-yillarning oxirlarida boshlandi. sperma kiti miyoglobin tomonidan Ser Jon Kovderi Kendryu,[89] u uchun u bilan bo'lishdi Kimyo bo'yicha Nobel mukofoti bilan Maks Peruts 1962 yilda.[90] Ushbu muvaffaqiyatdan so'ng oqsillar, nuklein kislotalar va boshqa biologik molekulalarning 130 mingdan ziyod rentgen-kristalli tuzilmalari aniqlandi.[91] Tahlil qilinadigan tuzilmalar soni bo'yicha eng yaqin raqobatlashuvchi usul yadro magnit-rezonans (NMR) spektroskopiyasi, bu o'ndan bir qismidan kamini hal qildi.[92] Kristalografiya o'zboshimchalik bilan katta molekulalarning tuzilishini hal qilishi mumkin, eritma holati NMR esa nisbatan kichiklari (70 k dan kam) bilan cheklangan.Da ). Farmatsevtik preparat oqsil nishoniga qanday ta'sir qilishini va qanday o'zgarishlar yaxshilanishi mumkinligini aniqlash uchun rentgen kristalografiyasi muntazam ravishda qo'llaniladi.[93] Biroq, ichki membrana oqsillari kristallanish qiyin bo'lib qoladi, chunki ular yuvish vositalarini yoki boshqalarni talab qiladi denaturantlar ularni alohida-alohida eritib olish uchun va bunday yuvish vositalari ko'pincha kristallashishga xalaqit beradi. Membran oqsillari .ning katta qismidir genom kabi katta fiziologik ahamiyatga ega ko'plab oqsillarni o'z ichiga oladi ion kanallari va retseptorlari.[94][95] Geliy kriyogenikasi oqsil kristallarida nurlanish shikastlanishining oldini olish uchun ishlatiladi.[96]
O'lchov o'lchovining boshqa uchida, hatto nisbatan kichik molekulalar ham rentgen kristallografiyasining kuchini hal qilishda qiyinchiliklar tug'dirishi mumkin. 1991 yilda dengiz organizmidan ajratilgan antibiotikga berilgan tuzilish, diazonamid A (C40H34Cl2N6O6, molar massa 765,65 g / mol), tuzilishining klassik isboti bilan noto'g'ri ekanligi isbotlandi: sintetik namuna tabiiy mahsulot bilan bir xil emas edi. Xato rentgen-kristallografiyasining to'g'ri -OH / -NH va o'zaro almashtirilgan -NH ni ajrata olmasligi bilan bog'liq edi.2 / -O- noto'g'ri tuzilgan guruhlar.[97] Asbobsozlik sohasida erishilgan yutuqlar bilan, shunga o'xshash guruhlarni zamonaviy bitta kristalli rentgen diffraktometrlari yordamida ajratish mumkin.
In bebaho vosita bo'lishiga qaramay tarkibiy biologiya, oqsil kristallografiyasi o'z metodologiyasida ma'lumotlarni izohlashga xalaqit beradigan ba'zi bir o'ziga xos muammolarni keltirib chiqaradi. Kristallanish jarayonida hosil bo'lgan kristall panjarada kristallga zich va nosimmetrik tarzda o'ralgan ko'plab tozalangan oqsil birliklari mavjud. Oldindan noma'lum bo'lgan oqsilni izlashda uning shakli va kristall panjaraning chegaralarini aniqlash qiyin bo'lishi mumkin. Oqsillar odatda kichikroq bo'linmalardan iborat bo'lib, kichik birliklarni ajratish va haqiqiy oqsilni aniqlash vazifasi hatto tajribali kristallograflar uchun ham qiyin bo'lishi mumkin. Kristallanish jarayonida yuzaga keladigan biologik bo'lmagan interfeyslar kristalli qadoqlash kontaktlari (yoki shunchaki, kristalli kontaktlar) deb nomlanadi va ularni kristallografik vositalar bilan ajratib bo'lmaydi. Qachon yangi oqsil tuzilishi rentgen kristallografiyasi bilan echilib, tarkibida yotadi Protein ma'lumotlar banki, uning mualliflaridan funktsional, biologik ahamiyatga ega bo'lgan oqsilni tashkil etadigan "biologik assambleya" ni belgilashlari so'raladi. Biroq, ma'lumotlarni yuborish paytida xatolar, etishmayotgan ma'lumotlar va noto'g'ri izohlar, tushunarsiz tuzilmalarni keltirib chiqaradi va ma'lumotlar bazasining ishonchliligini buzadi. Noto'g'ri izohlarda xatolik darajasi faqatgina 6,6% ga ko'tarilganligi haqida xabar berilgan[98] yoki taxminan 15%,[99] depozitga qo'yilgan inshootlar sonini hisobga olgan holda, ahamiyatsiz bo'lmagan o'lcham. Ushbu "interfeyslarni tasniflash muammosi" odatda hisoblash yondashuvlari bilan hal qilinadi va tan olingan mavzuga aylandi tarkibiy bioinformatika.
Tarqoqlik texnikasi
Elastik va noelastik sochilish
X-nurli kristallografiya - bu shakl elastik tarqalish; chiqayotgan rentgen nurlari xuddi shu energiyaga va shu bilan to'lqin uzunligiga, kiruvchi rentgen nurlari singari, faqat yo'nalishi o'zgargan. Aksincha, noaniq tarqalish energiya kiruvchi rentgen nuridan kristallga, masalan, ichki qobiq elektronini hayajonlanib yuqori darajaga o'tkazilganda sodir bo'ladi. energiya darajasi. Bunday noelastik tarqalish chiqayotgan nurning energiyasini pasaytiradi (yoki to'lqin uzunligini oshiradi). Elastik bo'lmagan tarqalish moddaning bunday qo'zg'alishini tekshirish uchun foydalidir, ammo materiyada tarqaladigan moddalarning tarqalishini aniqlashda emas, bu rentgen kristallografiyasining maqsadi.
X-nurlari to'lqin uzunligi 10 dan 0,01 gacha nanometrlar; kristallografiya uchun ishlatiladigan odatdagi to'lqin uzunligi 1 ga tengÅ (0,1 nm),[100] bu kovalent miqyosida kimyoviy aloqalar va bitta atomning radiusi. Uzunroq to'lqin uzunlikdagi fotonlar (masalan ultrabinafsha nurlanish ) atom holatini aniqlash uchun etarli rezolyutsiyaga ega bo'lmaydi. Kabi boshqa ekstremal, qisqa to'lqin uzunlikdagi fotonlar gamma nurlari ko'p sonli ishlab chiqarish qiyin, diqqatni jamlash qiyin va moddalar bilan juda qattiq ta'sir o'tkazib, hosil qiladi zarracha-zarracha juftliklari. Shuning uchun rentgen nurlari atomlarning rezolyutsiya tuzilmalarini aniqlashda to'lqin uzunligi uchun "shirinlik" dir. elektromagnit nurlanish.
Boshqa rentgen texnikasi
Bir kristalli difraksiyadan tashqari, elastik rentgen nurlanishining boshqa shakllari ham kiradi chang difraksiyasi, Kichik burchakli rentgen tarqalishi (SAXS ) va bir necha turdagi rentgen nurlari tolaning difraksiyasi tomonidan ishlatilgan Rosalind Franklin ni aniqlashda ikki spiralli tuzilish ning DNK. Umuman olganda, bitta kristalli rentgen diffraktsiyasi ushbu boshqa texnikalarga qaraganda ko'proq tarkibiy ma'lumotlarni taqdim etadi; ammo, u har doim ham mavjud bo'lmagan etarlicha katta va muntazam kristalni talab qiladi.
Ushbu tarqalish usullari odatda qo'llaniladi monoxromatik Kichkina og'ishlar bilan bitta to'lqin uzunligi bilan cheklangan rentgen nurlari. X-nurlarining keng spektridan (ya'ni turli xil to'lqin uzunlikdagi rentgen nurlari aralashmasidan), shuningdek, Laue usuli deb nomlanuvchi usulni rentgen diffraktsiyasini amalga oshirish uchun foydalanish mumkin. Bu rentgen difraksiyasining asl kashfiyotida qo'llanilgan usul. Laue tarqalishi juda qisqa vaqt ichida rentgen nurlari ta'sirida juda ko'p tizimli ma'lumotlarni beradi va shuning uchun juda tezkor hodisalarni tizimli tadqiq qilishda foydalaniladi (Vaqt aniqlangan kristallografiya ). Shu bilan birga, u kristalning to'liq atom tuzilishini aniqlash uchun monoxromatik tarqalish kabi juda mos kelmaydi va shuning uchun nisbatan oddiy atomli kristallar bilan yaxshi ishlaydi.
Laue orqasida aks ettirish rejimi keng spektr manbasidan orqaga taralgan rentgen nurlarini qayd etadi. Agar namuna rentgen nurlari o'tishi uchun juda qalin bo'lsa, bu foydali bo'ladi. Kristaldagi difraksion tekisliklar normal difraksion tekislikka tushayotgan nur va difraksiyalangan nur orasidagi burchakni ikkiga bo'lishini bilish orqali aniqlanadi. A Greninger jadvali foydalanish mumkin[101] orqa aks Laue fotosuratini talqin qilish.
Elektron va neytron difraksiyasi
Boshqa zarralar, masalan, elektronlar va neytronlar, ishlab chiqarish uchun ishlatilishi mumkin difraktsiya naqshlari. Elektronlar, neytronlar va rentgen nurlarining tarqalishi har xil fizik jarayonlarga asoslangan bo'lsa-da, hosil bo'lgan difraktsiya naqshlari bir xil yordamida tahlil qilinadi izchil difraksiyani tasvirlash texnikasi.
Quyida keltirilganidek, kristall ichidagi elektron zichligi va difraktsiya naqshlari oddiy matematik usul bilan bog'liq Furye konvertatsiyasi, bu zichlikni naqshlardan nisbatan osonlik bilan hisoblash imkonini beradi. Biroq, bu faqat tarqalish bo'lsa ishlaydi zaif, ya'ni sochilgan nurlar kiruvchi nurga qaraganda ancha kam intensiv bo'lsa. Zaif taralgan nurlar ikkinchi tarqalish hodisasiga duch kelmasdan kristalning qolgan qismidan o'tadi. Bunday qayta tarqalgan to'lqinlar "ikkilamchi tarqalish" deb nomlanadi va tahlilga to'sqinlik qiladi. Har qanday etarlicha qalin kristall ikkilamchi sochilishni keltirib chiqaradi, ammo rentgen nurlari elektronlar bilan nisbatan ozroq ta'sir o'tkazganligi sababli, bu umuman ahamiyatli emas. Aksincha, elektron nurlari nisbatan ingichka (> 100 nm) kristallar uchun ham kuchli ikkilamchi tarqalishni keltirib chiqarishi mumkin. Ushbu qalinlik ko'pchilikning diametriga to'g'ri kelganligi sababli viruslar, istiqbolli yo'nalish - bu ajratilgan elektron difraksiyasi makromolekulyar birikmalar, kabi virusli kapsidlar va molekulyar mashinalar krio- bilan amalga oshirilishi mumkin.elektron mikroskop. Bundan tashqari, elektronlarning moddalar bilan kuchli o'zaro ta'siri (rentgen nurlaridan 1000 baravar kuchliroq) juda kichik hajmdagi atom tuzilishini aniqlashga imkon beradi. Uchun arizalar maydoni elektron kristallografiyasi organik ingichka plyonkalar ustidagi membrana oqsillari kabi bio molekulalardan tortib (nanokristalli) intermetalik birikmalar va seolitlarning murakkab tuzilmalariga qadar.
Neytronlarning difraksiyasi - bu strukturani aniqlash uchun juda yaxshi usuldir, garchi etarli miqdorda neytronlarning monoxromatik nurlarini olish qiyin bo'lgan. An'anaga ko'ra, yadro reaktorlari neytronlarni ishlab chiqaradigan manbalar tomonidan ishlatilgan bo'lsa-da, ishlatilgan chayqalish tobora ko'proq mavjud bo'lib kelmoqda. Zaryadsiz bo'lgan neytronlar elektronlardan emas, balki atom yadrolaridan juda tez tarqaladi. Shuning uchun, neytronlarning tarqalishi, ayniqsa, kam elektronli yorug'lik atomlarining holatini kuzatish uchun juda foydali vodorod, bu asosan rentgen difraksiyasida ko'rinmaydi. Neytron tarqalishi, shuningdek, odatdagi nisbatni sozlash orqali erituvchini ko'rinmas holga keltiradigan ajoyib xususiyatga ega. suv, H2O, va og'ir suv, D.2O.
Usullari
Bir kristalli rentgen difraksiyasiga umumiy nuqtai

Rentgenning eng qadimgi va aniq usuli kristallografiya bu bir kristalli rentgen difraksiyasi, unda rentgen nurlari bitta kristallga urilib, tarqoq nurlarni hosil qiladi. Ular plyonka yoki boshqa detektorga tushganda, bu nurlar a difraktsiya naqshlari dog'lar; kristall asta-sekin aylanayotganda, bu nurlarning kuchli va burchaklari qayd etiladi.[102] Har bir nuqta a deb nomlanadi aks ettirish, chunki u rentgen nurlarining kristall ichidagi bir tekis joylashgan tekislik to'plamidan aks etishiga to'g'ri keladi. Etarli darajada toza va muntazam bo'lgan yagona kristallar uchun rentgen diffraksiyasi ma'lumotlari angstromning bir necha mingdan bir qismigacha va a ning o'ndan bir qismigacha bo'lgan o'rtacha kimyoviy bog'lanish uzunliklarini va burchaklarini aniqlashi mumkin. daraja navbati bilan. Kristall tarkibidagi atomlar statik emas, lekin ularning o'rtacha pozitsiyalari atrofida tebranadi, odatda angstromning o'ndan bir qismidan kamroq. Rentgen kristalografiyasi bu tebranishlarning hajmini o'lchashga imkon beradi.
Jarayon
Bir kristalli rentgen kristallografiyasining texnikasi uchta asosiy bosqichga ega. Birinchi va ko'pincha eng qiyin bosqich - o'rganilayotgan materialning etarli kristalini olishdir. Kristall etarlicha katta bo'lishi kerak (odatda barcha o'lchamlarda 0,1 mm dan katta), tarkibi toza va tuzilishi muntazam, ichki ko'rinishga ega bo'lmagan kamchiliklar singari yoriqlar yoki egizak.
Ikkinchi bosqichda kristall rentgen nurlarining kuchli nuriga, odatda bitta to'lqin uzunligiga joylashtirilgan (monoxromatik rentgen nurlari), aks ettirishning muntazam naqshini ishlab chiqarish. Difraksiyalangan rentgen nurlarining burchaklari va intensivligi o’lchanadi, har bir birikma noyob difraktsiya naqshiga ega.[103] Kristall asta-sekin aylanayotganda avvalgi ko'zgular yo'qoladi va yangilari paydo bo'ladi; kristallning har bir yo'nalishida har bir dog 'intensivligi qayd qilinadi. Bir nechta ma'lumotlar to'plamini to'plash kerak bo'lishi mumkin, ularning har bir to'plami kristalning to'liq aylanishining yarmidan ko'pini o'z ichiga oladi va odatda o'n minglab aks ettirishlarni o'z ichiga oladi.
Uchinchi bosqichda ushbu ma'lumotlar hisoblashda qo'shimcha kimyoviy ma'lumotlar bilan birlashtirilib, kristall ichidagi atomlarning joylashish modelini ishlab chiqaradi va takomillashtiradi. Atom tuzilishining yakuniy, takomillashtirilgan modeli - endi a kristall tuzilishi - odatda ochiq ma'lumotlar bazasida saqlanadi.
Cheklovlar
Kristalning takrorlanadigan birligi, uning birlik hujayrasi tobora kattalashib borar ekan, rentgen kristallografiyasi bilan ta'minlangan atom darajasidagi rasm ma'lum miqdordagi kuzatilgan aks ettirish uchun unchalik yaxshi aniqlanmagan (ko'proq "loyqa") bo'ladi. Rentgen nurlanishining ikki cheklovchi holati - "kichik molekula" (unga doimiy noorganik qattiq moddalar kiradi) va "makromolekulyar" kristallografiya ko'pincha aniqlanadi. Kichik molekulali kristallografiya odatda 100 dan kam atomli kristallarni o'z ichiga oladi assimetrik birlik; bunday kristalli tuzilmalar odatda juda yaxshi echilganki, atomlarni elektron zichligining ajratilgan "pufakchalari" deb bilish mumkin. Aksincha, makromolekulyar kristallografiya ko'pincha birlik hujayrasidagi o'n minglab atomlarni o'z ichiga oladi. Bunday kristall tuzilmalar odatda unchalik yaxshi echilmagan (ko'proq "bulg'angan"); atomlar va kimyoviy bog'lanishlar izolyatsiya qilingan atomlar kabi emas, balki elektron zichligi naychalari kabi ko'rinadi. Umuman olganda, kichik molekulalarni kristallashtirish ham makromolekulalarga qaraganda osonroq; ammo rentgen kristallografiyasi hatto uchun ham isbotlangan viruslar va takomillashtirilgan kristalografik tasvir va texnologiya orqali yuz minglab atomlarga ega oqsillar.[104] Odatda rentgen kristallografiyasini faqat namuna kristall shaklida bo'lgan taqdirda amalga oshirish mumkin bo'lsa-da, namunalarning kristal bo'lmagan shakllaridan namuna olish bo'yicha yangi tadqiqotlar o'tkazildi.[105]
Kristallanish

Kristallografiya nopok yoki notekis kristaldagi buzuqlikni tavsiflash uchun ishlatilishi mumkin bo'lsa-da, kristallografiya odatda atomlarning murakkab joylashuvi tuzilishini hal qilish uchun yuqori qonuniyatli sof kristalni talab qiladi. Ba'zan sof, muntazam kristallarni tabiiy yoki sintetik materiallardan olish mumkin, masalan metallar, minerals or other macroscopic materials. The regularity of such crystals can sometimes be improved with macromolecular crystal tavlash[106][107][108] va boshqa usullar. However, in many cases, obtaining a diffraction-quality crystal is the chief barrier to solving its atomic-resolution structure.[109]
Small-molecule and macromolecular crystallography differ in the range of possible techniques used to produce diffraction-quality crystals. Small molecules generally have few degrees of conformational freedom, and may be crystallized by a wide range of methods, such as kimyoviy bug 'cho'kmasi va qayta kristallanish. By contrast, macromolecules generally have many degrees of freedom and their crystallization must be carried out so as to maintain a stable structure. For example, proteins and larger RNK molecules cannot be crystallized if their tertiary structure has been ochildi; therefore, the range of crystallization conditions is restricted to solution conditions in which such molecules remain folded.
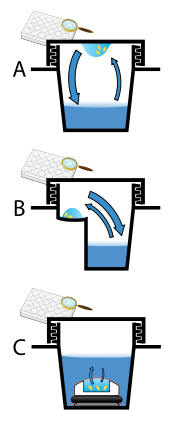
Protein crystals are almost always grown in solution. The most common approach is to lower the solubility of its component molecules very gradually; if this is done too quickly, the molecules will precipitate from solution, forming a useless dust or amorphous gel on the bottom of the container. Crystal growth in solution is characterized by two steps: yadrolanish of a microscopic crystallite (possibly having only 100 molecules), followed by o'sish of that crystallite, ideally to a diffraction-quality crystal.[110][111] The solution conditions that favor the first step (nucleation) are not always the same conditions that favor the second step (subsequent growth). The crystallographer's goal is to identify solution conditions that favor the development of a single, large crystal, since larger crystals offer improved resolution of the molecule. Consequently, the solution conditions should disfavor the first step (nucleation) but yaxshilik the second (growth), so that only one large crystal forms per droplet. If nucleation is favored too much, a shower of small crystallites will form in the droplet, rather than one large crystal; if favored too little, no crystal will form whatsoever. Other approaches involves, crystallizing proteins under oil, where aqueous protein solutions are dispensed under liquid oil, and water evaporates through the layer of oil. Different oils have different evaporation permeabilities, therefore yielding changes in concentration rates from different percipient/protein mixture.[112]
It is extremely difficult to predict good conditions for nucleation or growth of well-ordered crystals.[113] In practice, favorable conditions are identified by skrining; a very large batch of the molecules is prepared, and a wide variety of crystallization solutions are tested.[114] Hundreds, even thousands, of solution conditions are generally tried before finding the successful one. The various conditions can use one or more physical mechanisms to lower the solubility of the molecule; for example, some may change the pH, some contain salts of the Hofmeister seriyasi or chemicals that lower the dielectric constant of the solution, and still others contain large polymers such as polietilen glikol that drive the molecule out of solution by entropic effects. It is also common to try several temperatures for encouraging crystallization, or to gradually lower the temperature so that the solution becomes supersaturated. These methods require large amounts of the target molecule, as they use high concentration of the molecule(s) to be crystallized. Due to the difficulty in obtaining such large quantities (milligramm ) of crystallization-grade protein, robots have been developed that are capable of accurately dispensing crystallization trial drops that are in the order of 100 nanolitrlar hajmda. This means that 10-fold less protein is used per experiment when compared to crystallization trials set up by hand (in the order of 1 microliter ).[115]
Several factors are known to inhibit or mar crystallization. The growing crystals are generally held at a constant temperature and protected from shocks or vibrations that might disturb their crystallization. Impurities in the molecules or in the crystallization solutions are often inimical to crystallization. Conformational flexibility in the molecule also tends to make crystallization less likely, due to entropy. Molecules that tend to self-assemble into regular helices are often unwilling to assemble into crystals.[iqtibos kerak ] Crystals can be marred by egizak, which can occur when a unit cell can pack equally favorably in multiple orientations; although recent advances in computational methods may allow solving the structure of some twinned crystals. Having failed to crystallize a target molecule, a crystallographer may try again with a slightly modified version of the molecule; even small changes in molecular properties can lead to large differences in crystallization behavior.
Ma'lumot yig'ish
Mounting the crystal
The crystal is mounted for measurements so that it may be held in the X-ray beam and rotated. There are several methods of mounting. In the past, crystals were loaded into glass capillaries with the crystallization solution (the ona likyor ). Nowadays, crystals of small molecules are typically attached with oil or glue to a glass fiber or a loop, which is made of nylon or plastic and attached to a solid rod. Protein crystals are scooped up by a loop, then flash-frozen with suyuq azot.[116] This freezing reduces the radiation damage of the X-rays, as well as the noise in the Bragg peaks due to thermal motion (the Debye-Waller effect). However, untreated protein crystals often crack if flash-frozen; therefore, they are generally pre-soaked in a cryoprotectant solution before freezing.[117] Unfortunately, this pre-soak may itself cause the crystal to crack, ruining it for crystallography. Generally, successful cryo-conditions are identified by trial and error.
The capillary or loop is mounted on a goniometr, which allows it to be positioned accurately within the X-ray beam and rotated. Since both the crystal and the beam are often very small, the crystal must be centered within the beam to within ~25 micrometers accuracy, which is aided by a camera focused on the crystal. The most common type of goniometer is the "kappa goniometer", which offers three angles of rotation: the ω angle, which rotates about an axis perpendicular to the beam; the κ angle, about an axis at ~50° to the ω axis; and, finally, the φ angle about the loop/capillary axis. When the κ angle is zero, the ω and φ axes are aligned. The κ rotation allows for convenient mounting of the crystal, since the arm in which the crystal is mounted may be swung out towards the crystallographer. The oscillations carried out during data collection (mentioned below) involve the ω axis only. An older type of goniometer is the four-circle goniometer, and its relatives such as the six-circle goniometer.
Rentgen manbalari
Rotating anode
Small scale crystallography can be done with a local Rentgen naychasi source, typically coupled with an image plate detektor. These have the advantage of being relatively inexpensive and easy to maintain, and allow for quick screening and collection of samples. However, the wavelength of the light produced is limited by the availability of different anod materiallar. Furthermore, the intensity is limited by the power applied and cooling capacity available to avoid melting the anode. In such systems, electrons are boiled off of a cathode and accelerated through a strong electric potential of ~50kV; having reached a high speed, the electrons collide with a metal plate, emitting dilshodbek and some strong spectral lines corresponding to the excitation of inner-shell electrons metall. The most common metal used is mis, which can be kept cool easily, due to its high issiqlik o'tkazuvchanligi, and which produces strong Ka va Kβ chiziqlar. Kβ line is sometimes suppressed with a thin (~10 µm) nickel foil. The simplest and cheapest variety of sealed X-ray tube has a stationary anode (the Crookes tube ) and run with ~2 kVt of electron beam power. The more expensive variety has a rotating-anode type source that run with ~14 kW of e-beam power.
X-rays are generally filtered (by use of Rentgen filtrlari ) to a single wavelength (made monochromatic) and kollimatsiya qilingan to a single direction before they are allowed to strike the crystal. The filtering not only simplifies the data analysis, but also removes radiation that degrades the crystal without contributing useful information. Collimation is done either with a collimator (basically, a long tube) or with a clever arrangement of gently curved mirrors. Mirror systems are preferred for small crystals (under 0.3 mm) or with large unit cells (over 150 Å).
Rotating anodes were used by Joanna (Joka) Maria Vandenberg in the first experiments[118][119] that demonstrated the power of X rays for quick (in real time production) screening of large InGaAsP thin film wafers for sifat nazorati ning quantum well lasers.
Sinxrotron nurlanishi
Sinxrotron nurlanishi sources are some of the brightest light sources on earth and are some of the most powerful tools available to X-ray crystallographers. X-ray beams generated in large machines called sinxrotronlar which accelerate electrically charged particles, often electrons, to nearly the speed of light and confine them in a (roughly) circular loop using magnetic fields.
Synchrotrons are generally national facilities, each with several dedicated nurli chiziqlar where data is collected without interruption. Synchrotrons were originally designed for use by high-energy physicists studying subatomik zarralar va kosmik hodisalar. The largest component of each synchrotron is its electron storage ring. This ring is actually not a perfect circle, but a many-sided polygon. At each corner of the polygon, or sector, precisely aligned magnets bend the electron stream. As the electrons' path is bent, they emit bursts of energy in the form of X-rays.
Using synchrotron radiation frequently has specific requirements for X-ray crystallography. Shiddatli ionlashtiruvchi nurlanish sabab bo'lishi mumkin radiatsiya shikastlanishi to samples, particularly macromolecular crystals. Cryo crystallography protects the sample from radiation damage, by freezing the crystal at suyuq azot temperatures (~100 K ).[120] However, synchrotron radiation frequently has the advantage of user-selectable wavelengths, allowing for anormal tarqalish experiments which maximizes anomalous signal. This is critical in experiments such as SAD va TELBA.
Erkin elektronli lazer
Erkin elektronli lazerlar have been developed for use in X-ray crystallography.[121] These are the brightest X-ray sources currently available; with the X-rays coming in femtosekundiya bursts. The intensity of the source is such that atomic resolution diffraction patterns can be resolved for crystals otherwise too small for collection. However, the intense light source also destroys the sample,[122] requiring multiple crystals to be shot. As each crystal is randomly oriented in the beam, hundreds of thousands of individual diffraction images must be collected in order to get a complete data set. This method, serial femtosecond crystallography, has been used in solving the structure of a number of protein crystal structures, sometimes noting differences with equivalent structures collected from synchrotron sources.[123]
Recording the reflections

When a crystal is mounted and exposed to an intense beam of X-rays, it scatters the X-rays into a pattern of spots or aks ettirishlar that can be observed on a screen behind the crystal. A similar pattern may be seen by shining a lazer ko'rsatkichi a ixcham disk. The relative intensities of these spots provide the information to determine the arrangement of molecules within the crystal in atomic detail. The intensities of these reflections may be recorded with fotografik film, an area detector (such as a pixel detector ) yoki bilan zaryad bilan bog'langan qurilma (CCD) tasvir sensori. The peaks at small angles correspond to low-resolution data, whereas those at high angles represent high-resolution data; thus, an upper limit on the eventual resolution of the structure can be determined from the first few images. Some measures of diffraction quality can be determined at this point, such as the mozaika of the crystal and its overall disorder, as observed in the peak widths. Some pathologies of the crystal that would render it unfit for solving the structure can also be diagnosed quickly at this point.
One image of spots is insufficient to reconstruct the whole crystal; it represents only a small slice of the full Fourier transform. To collect all the necessary information, the crystal must be rotated step-by-step through 180°, with an image recorded at every step; actually, slightly more than 180° is required to cover reciprocal space, due to the curvature of the Evald shar. However, if the crystal has a higher symmetry, a smaller angular range such as 90° or 45° may be recorded. The rotation axis should be changed at least once, to avoid developing a "blind spot" in reciprocal space close to the rotation axis. It is customary to rock the crystal slightly (by 0.5–2°) to catch a broader region of reciprocal space.
Multiple data sets may be necessary for certain phasing methods. For example, MAD phasing requires that the scattering be recorded at least three (and usually four, for redundancy) wavelengths of the incoming X-ray radiation. A single crystal may degrade too much during the collection of one data set, owing to radiation damage; in such cases, data sets on multiple crystals must be taken.[124]
Ma'lumotlarni tahlil qilish
Crystal symmetry, unit cell, and image scaling
The recorded series of two-dimensional diffraction patterns, each corresponding to a different crystal orientation, is converted into a three-dimensional model of the electron density; the conversion uses the mathematical technique of Fourier transforms, which is explained quyida. Each spot corresponds to a different type of variation in the electron density; the crystallographer must determine qaysi variation corresponds to qaysi spot (indeksatsiya), the relative strengths of the spots in different images (merging and scaling) and how the variations should be combined to yield the total electron density (bosqichma-bosqich).
Data processing begins with indeksatsiya the reflections. This means identifying the dimensions of the unit cell and which image peak corresponds to which position in reciprocal space. A byproduct of indexing is to determine the symmetry of the crystal, i.e., its kosmik guruh. Some space groups can be eliminated from the beginning. For example, reflection symmetries cannot be observed in chiral molecules; thus, only 65 space groups of 230 possible are allowed for protein molecules which are almost always chiral. Indexing is generally accomplished using an autoindexing muntazam.[125] Having assigned symmetry, the data is then birlashtirilgan. This converts the hundreds of images containing the thousands of reflections into a single file, consisting of (at the very least) records of the Miller indeksi of each reflection, and an intensity for each reflection (at this state the file often also includes error estimates and measures of partiality (what part of a given reflection was recorded on that image)).
A full data set may consist of hundreds of separate images taken at different orientations of the crystal. The first step is to merge and scale these various images, that is, to identify which peaks appear in two or more images (birlashma) and to scale the relative images so that they have a consistent intensity scale. Optimizing the intensity scale is critical because the relative intensity of the peaks is the key information from which the structure is determined. The repetitive technique of crystallographic data collection and the often high symmetry of crystalline materials cause the diffractometer to record many symmetry-equivalent reflections multiple times. This allows calculating the symmetry-related R-omil, a reliability index based upon how similar are the measured intensities of symmetry-equivalent reflections,[tushuntirish kerak ] thus assessing the quality of the data.
Initial phasing
The data collected from a diffraction experiment is a reciprocal space representation of the crystal lattice. The position of each diffraction 'spot' is governed by the size and shape of the unit cell, and the inherent simmetriya within the crystal. The intensity of each diffraction 'spot' is recorded, and this intensity is proportional to the square of the tuzilish omili amplituda. The tuzilish omili a murakkab raqam containing information relating to both the amplituda va bosqich a to'lqin. In order to obtain an interpretable electron density map, both amplitude and phase must be known (an electron density map allows a crystallographer to build a starting model of the molecule). The phase cannot be directly recorded during a diffraction experiment: this is known as the faza muammosi. Initial phase estimates can be obtained in a variety of ways:
- Ab initio bosqichma-bosqich yoki to'g'ridan-to'g'ri usullar – This is usually the method of choice for small molecules (<1000 non-hydrogen atoms), and has been used successfully to solve the phase problems for small proteins. If the resolution of the data is better than 1.4 Å (140 pm ), to'g'ridan-to'g'ri usullar can be used to obtain phase information, by exploiting known phase relationships between certain groups of reflections.[126][127]
- Molekulyar almashtirish – if a related structure is known, it can be used as a search model in molecular replacement to determine the orientation and position of the molecules within the unit cell. The phases obtained this way can be used to generate electron density maps.[128]
- Anomal rentgen tarqalishi (TELBA yoki SAD phasing ) – the X-ray wavelength may be scanned past an absorption edge[qachon aniqlanadi? ] of an atom, which changes the scattering in a known way. By recording full sets of reflections at three different wavelengths (far below, far above and in the middle of the absorption edge) one can solve for the substructure of the anomalously diffracting atoms and hence the structure of the whole molecule. The most popular method of incorporating anomalous scattering atoms into proteins is to express the protein in a metionin auxotroph (a host incapable of synthesizing methionine) in a media rich in seleno-methionine, which contains selen atomlar A MAD experiment can then be conducted around the absorption edge, which should then yield the position of any methionine residues within the protein, providing initial phases.[129]
- Heavy atom methods (multiple isomorphous replacement ) – If electron-dense metal atoms can be introduced into the crystal, to'g'ridan-to'g'ri usullar yoki Patterson-space methods can be used to determine their location and to obtain initial phases. Such heavy atoms can be introduced either by soaking the crystal in a heavy atom-containing solution, or by co-crystallization (growing the crystals in the presence of a heavy atom). As in MAD phasing, the changes in the scattering amplitudes can be interpreted to yield the phases. Although this is the original method by which protein crystal structures were solved, it has largely been superseded by MAD phasing with selenomethionine.[128]
Model building and phase refinement


Having obtained initial phases, an initial model can be built. The atomic positions in the model and their respective Debye-Waller factors (yoki B-factors, accounting for the thermal motion of the atom) can be refined to fit the observed diffraction data, ideally yielding a better set of phases. A new model can then be fit to the new electron density map and successive rounds of refinement is carried out. This interative process continues until the correlation between the diffraction data and the model is maximized. The agreement is measured by an R- omil sifatida belgilangan
qayerda F bo'ladi tuzilish omili. A similar quality criterion is Rozod, which is calculated from a subset (~10%) of reflections that were not included in the structure refinement. Ikkalasi ham R factors depend on the resolution of the data. Qoida tariqasida, Rozod should be approximately the resolution in angstroms divided by 10; thus, a data-set with 2 Å resolution should yield a final Rozod ~ 0.2. Chemical bonding features such as stereochemistry, hydrogen bonding and distribution of bond lengths and angles are complementary measures of the model quality. Phase bias is a serious problem in such iterative model building. Omit maps are a common technique used to check for this.[tushuntirish kerak ]
It may not be possible to observe every atom in the asymmetric unit. Ko'p hollarda, Crystallographic disorder smears the electron density map. Weakly scattering atoms such as hydrogen are routinely invisible. It is also possible for a single atom to appear multiple times in an electron density map, e.g., if a protein sidechain has multiple (<4) allowed conformations. In still other cases, the crystallographer may detect that the covalent structure deduced for the molecule was incorrect, or changed. For example, proteins may be cleaved or undergo post-translational modifications that were not detected prior to the crystallization.
Buzuqlik
A common challenge in refinement of crystal structures results from crystallographic disorder. Disorder can take many forms but in general involves the coexistence of two or more species or conformations. Failure to recognize disorder results in flawed interpretation. Pitfalls from improper modeling of disorder are illustrated by the discounted hypothesis of bond stretch isomerism.[132] Disorder is modelled with respect to the relative population of the components, often only two, and their identity. In structures of large molecules and ions, solvent and counterions are often disordered.
Applied computational data analysis
The use of computational methods for the powder X-ray diffraction data analysis is now generalized. It typically compares the experimental data to the simulated diffractogram of a model structure, taking into account the instrumental parameters, and refines the structural or microstructural parameters of the model using eng kichik kvadratchalar based minimization algorithm. Most available tools allowing phase identification and structural refinement are based on the Rietveld usuli,[133][134] some of them being open and free software such as FullProf Suite,[135][136] Jana2006,[137] MAUD,[138][139][140] Rietan,[141] GSAS,[142] etc. while others are available under commercials licenses such as Diffrac.Suite TOPAS,[143] Match!,[144] etc. Most of these tools also allow Le garov refinement (also referred to as profile matching), that is, refinement of the cell parameters based on the Bragg peaks positions and peak profiles, without taking into account the crystallographic structure by itself. More recent tools allow the refinement of both structural and microstructural data, such as the FAULTS program included in the FullProf Suite,[145] which allows the refinement of structures with planar defects (e.g. stacking faults, twinnings, intergrowths).
Deposition of the structure
Once the model of a molecule's structure has been finalized, it is often deposited in a crystallographic database kabi Kembrijning tarkibiy ma'lumotlar bazasi (for small molecules), the Inorganic Crystal Structure Database (ICSD) (for inorganic compounds) or the Protein ma'lumotlar banki (for protein and sometimes nucleic acids). Many structures obtained in private commercial ventures to crystallize medicinally relevant proteins are not deposited in public crystallographic databases.
Diffraction theory
The main goal of X-ray crystallography is to determine the density of electrons f(r) throughout the crystal, where r represents the three-dimensional position vektor within the crystal. To do this, X-ray scattering is used to collect data about its Fourier transform F(q), which is inverted mathematically to obtain the density defined in real space, using the formula
qaerda ajralmas is taken over all values of q. The three-dimensional real vector q represents a point in reciprocal space, that is, to a particular oscillation in the electron density as one moves in the direction in which q ochkolar. Uzunligi q ga mos keladi divided by the wavelength of the oscillation. The corresponding formula for a Fourier transform will be used below
qaerda ajralmas is summed over all possible values of the position vector r within the crystal.
The Fourier transform F(q) odatda a murakkab raqam, and therefore has a kattalik |F(q) | va a bosqich φ(q) related by the equation
The intensities of the reflections observed in X-ray diffraction give us the magnitudes |F(q) | but not the phases φ(q). To obtain the phases, full sets of reflections are collected with known alterations to the scattering, either by modulating the wavelength past a certain absorption edge or by adding strongly scattering (i.e., electron-dense) metal atoms such as simob. Combining the magnitudes and phases yields the full Fourier transform F(q), which may be inverted to obtain the electron density f(r).
Crystals are often idealized as being Mukammal periodic. In that ideal case, the atoms are positioned on a perfect lattice, the electron density is perfectly periodic, and the Fourier transform F(q) is zero except when q ga tegishli o'zaro panjara (so'zda Bragg cho'qqilari). In reality, however, crystals are not perfectly periodic; atoms vibrate about their mean position, and there may be disorder of various types, such as mozaika, dislokatsiyalar, har xil nuqsonli nuqsonlar, and heterogeneity in the conformation of crystallized molecules. Therefore, the Bragg peaks have a finite width and there may be significant diffuse scattering, a continuum of scattered X-rays that fall between the Bragg peaks.
Intuitive understanding by Bragg's law
An intuitive understanding of X-ray diffraction can be obtained from the Bragg model of diffraction. In this model, a given reflection is associated with a set of evenly spaced sheets running through the crystal, usually passing through the centers of the atoms of the crystal lattice. The orientation of a particular set of sheets is identified by its three Miller indices (h, k, l), and let their spacing be noted by d. William Lawrence Bragg proposed a model in which the incoming X-rays are scattered specularly (mirror-like) from each plane; from that assumption, X-rays scattered from adjacent planes will combine constructively (konstruktiv aralashuv ) when the angle θ between the plane and the X-ray results in a path-length difference that is an integer multiple n of the X-ray wavelength λ.
A reflection is said to be indekslangan when its Miller indices (or, more correctly, its o'zaro panjara vector components) have been identified from the known wavelength and the scattering angle 2θ. Such indexing gives the unit-cell parameters, the lengths and angles of the unit-cell, as well as its kosmik guruh. Beri Bragg qonuni does not interpret the relative intensities of the reflections, however, it is generally inadequate to solve for the arrangement of atoms within the unit-cell; for that, a Fourier transform method must be carried out.
Scattering as a Fourier transform
The incoming X-ray beam has a polarization and should be represented as a vector wave; however, for simplicity, let it be represented here as a scalar wave. We also ignore the complication of the time dependence of the wave and just concentrate on the wave's spatial dependence. Samolyot to'lqinlari bilan ifodalanishi mumkin to'lqin vektori kyilda, and so the strength of the incoming wave at time t = 0 is given by
At position r within the sample, let there be a density of scatterers f(r); these scatterers should produce a scattered spherical wave of amplitude proportional to the local amplitude of the incoming wave times the number of scatterers in a small volume dV haqida r
qayerda S mutanosiblik doimiysi.
Consider the fraction of scattered waves that leave with an outgoing wave-vector of kchiqib and strike the screen at rekran. Since no energy is lost (elastic, not inelastic scattering), the wavelengths are the same as are the magnitudes of the wave-vectors |kyilda|=|kchiqib|. From the time that the photon is scattered at r until it is absorbed at rekran, the photon undergoes a change in phase
The net radiation arriving at rekran is the sum of all the scattered waves throughout the crystal
which may be written as a Fourier transform
qayerda q = kchiqib – kyilda. The measured intensity of the reflection will be square of this amplitude
Friedel and Bijvoet mates
For every reflection corresponding to a point q in the reciprocal space, there is another reflection of the same intensivlik at the opposite point -q. This opposite reflection is known as the Friedel mate of the original reflection. This symmetry results from the mathematical fact that the density of electrons f(r) at a position r har doim a haqiqiy raqam. Yuqorida ta'kidlab o'tilganidek, f(r) is the inverse transform of its Fourier transform F(q); however, such an inverse transform is a murakkab raqam umuman. Buni ta'minlash uchun f(r) is real, the Fourier transform F(q) must be such that the Friedel mates F(−q) va F(q) bor murakkab konjugatlar bir-birining. Shunday qilib, F(−q) has the same magnitude as F(q) but they have the opposite phase, i.e., φ(q) = −φ(q)
The equality of their magnitudes ensures that the Friedel mates have the same intensity |F|2. Ushbu simmetriya to'liq Furye konvertatsiyasini o'zaro fazoning atigi yarmidan o'lchashga imkon beradi, masalan, to'liq 360 ° aylanish o'rniga kristalni 180 ° dan bir oz ko'proq aylantirish orqali. Simmetriyasi sezilarli bo'lgan kristallarda aks ettirishlar bir xil intensivlikka ega bo'lishi mumkin (Bijvoet juftlari); bunday hollarda, hatto o'zaro bo'shliqning kamroq miqdorini o'lchash kerak bo'lishi mumkin. Yuqori simmetriyaning qulay holatlarida, o'zaro ta'sir doirasini to'liq o'rganish uchun ba'zida faqat 90 ° yoki hatto faqat 45 ° ma'lumotlar talab qilinadi.
Fridel-mate cheklovi teskari Furye konvertatsiyasi ta'rifidan kelib chiqishi mumkin
Beri Eyler formulasi emenx = cos (x) men gunoh qilaman (x), teskari Furye konversiyasini sof real qism va sof xayoliy qism yig'indisiga ajratish mumkin
Funktsiya f(r) agar ikkinchi integral bo'lsa va faqat shu holda haqiqiy bo'ladi Mengunoh ning barcha qiymatlari uchun nolga teng r. O'z navbatida, bu yuqoridagi cheklov qondirilgan taqdirdagina to'g'ri keladi
beri Mengunoh = −Mengunoh shuni anglatadiki Mengunoh = 0.
Evald shar
Har bir rentgen diffraktsion tasvir faqat tilimni, o'zaro fazoning sharsimon bo'lagini ifodalaydi, chunki Evald shar qurilishi bilan ko'rish mumkin. Ikkalasi ham kchiqib va kyilda to'lqin uzunligi o'zgarmaganligi sababli, elastik tarqalishi tufayli bir xil uzunlikka ega. Shuning uchun ular ichida joylashgan sharchada ikkita radiusli vektor sifatida ifodalanishi mumkin o'zaro bo'shliq, ning qiymatlarini ko'rsatadigan q berilgan diffraktsiya tasvirida namuna olingan. Kiruvchi rentgen nurlarining kiruvchi to'lqin uzunliklarida biroz tarqalish bo'lgani uchun, | qiymatlariF(q) faqat uchun o'lchanishi mumkin q o'sha radiuslarga mos keladigan ikkita shar o'rtasida joylashgan vektorlar. Shuning uchun, Furye konvertatsiyasi ma'lumotlarining to'liq to'plamini olish uchun kristalni 180 ° dan bir oz ko'proq aylantirish kerak, yoki agar etarli simmetriya mavjud bo'lsa, ba'zan kamroq bo'ladi. Haqiqiy funktsiyalarning (masalan, elektron zichligi) Furye o'zgarishiga xos bo'lgan simmetriya tufayli to'liq 360 ° burilish kerak emas, lekin berilgan rezolyutsiyada barcha o'zaro bo'shliqni qoplash uchun 180 ° dan "bir oz ko'proq" kerak egriligi Evald shar. Amalda kristal ozgina miqdorda (0,25-1 °) tebranadi, sharsimon Evald chig'anoqlari chegaralari yaqinidagi ko'zgularni o'z ichiga oladi.
Patterson funktsiyasi
Fourier konvertatsiyasining taniqli natijasi bu avtokorrelyatsiya avtokorrelyatsiya deyilgan teorema v(r) funktsiya f(r)
Fourier konvertatsiyasiga ega C(q) bu kvadrat kattaligi F(q)
Shuning uchun avtokorrelyatsiya funktsiyasi v(r) elektron zichligi (. nomi bilan ham tanilgan Patterson funktsiyasi[146]) fazalarni hisoblamasdan, aks ettirish intensivligidan to'g'ridan-to'g'ri hisoblash mumkin. Printsipial jihatdan, bu to'g'ridan-to'g'ri kristal tuzilishini aniqlash uchun ishlatilishi mumkin; ammo, amalda amalga oshirish qiyin. Avtokorrelyatsiya funktsiyasi ning taqsimlanishiga mos keladi vektorlar kristaldagi atomlar orasidagi; Shunday qilib, ning N uning birlik hujayrasidagi atomlar bo'lishi mumkin N(N - 1) Patterson funktsiyasida eng yuqori darajaga ko'tariladi. Zichliklarni o'lchashdagi muqarrar xatolar va atomlararo vektorlardan atom holatini tiklashdagi matematik qiyinchiliklarni hisobga olgan holda, bu uslub eng oddiy kristallardan tashqari tuzilmalarni echishda kamdan kam qo'llaniladi.
Kristalning afzalliklari
Aslida, atom tuzilishini kristal bo'lmagan namunalarga, hattoki bitta molekulaga rentgen nurlari sochilishini qo'llash orqali aniqlash mumkin edi. Biroq, ularning davriyligi tufayli kristallar ancha kuchli signal beradi. Kristalli namuna ta'rifi bo'yicha davriy; kristal ko'pchilardan tashkil topgan birlik hujayralari uchta mustaqil yo'nalishda cheksiz takrorlangan. Bunday davriy tizimlarda a Furye konvertatsiyasi deb nomlanuvchi o'zaro fazoda vaqti-vaqti bilan takrorlanadigan nuqtalarda to'plangan Bragg cho'qqilari; Bragg cho'qqilari difraksiya tasvirida kuzatilgan aks ettirish joylariga to'g'ri keladi. Ushbu aks ettirishdagi amplituda son bilan chiziqli ravishda o'sib borgani uchun N tarqaluvchilar, kuzatilgan intensivlik kabi dog'lar kvadratik ravishda o'sishi kerak N2. Boshqacha qilib aytganda, kristall yordamida alohida birlik hujayralarining kuchsiz tarqalishi shovqin ustida kuzatilishi mumkin bo'lgan ancha kuchliroq, izchil aks ettirishga jamlanadi. Bu misol konstruktiv aralashuv.
Suyuq, chang yoki amorf namunada ushbu namunadagi molekulalar tasodifiy yo'nalishda bo'ladi. Bunday namunalar doimiy ravishda Fourier spektriga ega bo'lib, u o'z amplitudasini bir tekisda tarqatadi va shu bilan o'lchangan signal intensivligini pasaytiradi. SAXS. Eng muhimi, yo'naltirilgan ma'lumot yo'qoladi. Nazariy jihatdan mumkin bo'lsa-da, bunday aylantirilgan o'rtacha ma'lumotlardan murakkab, assimetrik molekulalarning atom o'lchamlari tuzilmalarini olish tajribada qiyin. Qidiruv ish tolaning difraksiyasi unda kichik birliklar vaqti-vaqti bilan kamida bitta o'lchovda joylashtirilgan.
Rentgen kristallografiyasini o'z ichiga olgan Nobel mukofotlari
| Yil | Laureat | Mukofot | Mantiqiy asos |
|---|---|---|---|
| 1914 | Maks fon Laue | Fizika | "X-nurlarining kristallar bilan difraksiyasini kashf etgani uchun",[147] rivojlanishidagi muhim qadam Rentgen spektroskopiyasi. |
| 1915 | Uilyam Genri Bragg | Fizika | "Tahlildagi xizmatlari uchun kristall tuzilishi rentgen nurlari yordamida "[148] |
| 1915 | Uilyam Lourens Bragg | Fizika | "Tahlildagi xizmatlari uchun kristall tuzilishi rentgen nurlari yordamida "[148] |
| 1962 | Maks F. Perutz | Kimyo | "ning tuzilmalarini o'rganish uchun global oqsillar "[149] |
| 1962 | John C. Kendrew | Kimyo | "ning tuzilmalarini o'rganish uchun global oqsillar "[149] |
| 1962 | Jeyms Devi Uotson | Dori | "Ning molekulyar tuzilishiga oid kashfiyotlari uchun nuklein kislotalar va uning jonli materialda ma'lumot uzatishdagi ahamiyati "[150] |
| 1962 | Frensis Garri Kompton Krik | Dori | "Ning molekulyar tuzilishiga oid kashfiyotlari uchun nuklein kislotalar va uning jonli materialda ma'lumot uzatishdagi ahamiyati "[150] |
| 1962 | Moris Xyu Frederik Uilkins | Dori | "Ning molekulyar tuzilishiga oid kashfiyotlari uchun nuklein kislotalar va uning jonli materialda ma'lumot uzatishdagi ahamiyati "[150] |
| 1964 | Doroti Xodkin | Kimyo | "Uning uchun rentgen texnikasi bilan aniqlash muhim biokimyoviy moddalar tuzilishi "[151] |
| 1972 | Stenford Mur | Kimyo | "Ning faol markazining kimyoviy tuzilishi va katalitik faolligi o'rtasidagi bog'liqlikni tushunishga qo'shgan hissasi uchun ribonukleaz molekula "[152] |
| 1972 | Uilyam X.Shteyn | Kimyo | "Ning faol markazining kimyoviy tuzilishi va katalitik faolligi o'rtasidagi bog'liqlikni tushunishga qo'shgan hissasi uchun ribonukleaz molekula "[152] |
| 1976 | Uilyam N. Lipscomb | Kimyo | "Ning tuzilishi bo'yicha olib borgan tadqiqotlari uchun boran kimyoviy bog'lanish muammolarini yorituvchi "[153] |
| 1985 | Jerom Karle | Kimyo | "Rivojlanishdagi ulkan yutuqlari uchun to'g'ridan-to'g'ri usullar kristalli konstruksiyalarni aniqlash uchun "[154] |
| 1985 | Herbert A. Hauptman | Kimyo | "Rivojlanishdagi ulkan yutuqlari uchun to'g'ridan-to'g'ri usullar kristalli konstruksiyalarni aniqlash uchun "[154] |
| 1988 | Yoxann Deyzenxofer | Kimyo | "A ning uch o'lchovli tuzilishini aniqlash uchun fotosintezli reaktsiya markazi "[155] |
| 1988 | Xartmut Mishel | Kimyo | "A ning uch o'lchovli tuzilishini aniqlash uchun fotosintezli reaktsiya markazi "[155] |
| 1988 | Robert Xuber | Kimyo | "A ning uch o'lchovli tuzilishini aniqlash uchun fotosintezli reaktsiya markazi "[155] |
| 1997 | Jon E. Uoker | Kimyo | "Ularning yoritilganligi uchun fermentativ mexanizm adenozin trifosfat (ATP) sintezi asosida "[156] |
| 2003 | Roderik MakKinnon | Kimyo | "Hujayra membranalaridagi kanallarga oid kashfiyotlar uchun [...] strukturaviy va mexanik uchun ion kanallarini o'rganish "[157] |
| 2003 | Piter Agre | Kimyo | "Hujayra membranalaridagi kanallarga oid kashfiyotlar uchun [...] suv kanallari "[157] |
| 2006 | Rojer D. Kornberg | Kimyo | "Ning molekulyar asoslarini o'rgangani uchun eukaryotik transkripsiya "[158] |
| 2009 | Ada E. Yonat | Kimyo | "Ning tuzilishi va funktsiyasini o'rganish uchun ribosoma "[159] |
| 2009 | Tomas A. Shtayts | Kimyo | "Ning tuzilishi va funktsiyasini o'rganish uchun ribosoma "[159] |
| 2009 | Venkatraman Ramakrishnan | Kimyo | "Ning tuzilishi va funktsiyasini o'rganish uchun ribosoma "[159] |
| 2012 | Brayan Kobilka | Kimyo | "Tadqiqotlar uchun G-oqsil bilan bog'langan retseptorlari "[160] |
Ilovalar
Rentgen diffraktsiyasi kimyoviy, biokimyoviy, fizik, moddiy va mineralogiya fanlarida keng va turli xil qo'llanmalarga ega. Laue 1937 yilda ushbu uslub "mikroskop berganidan o'n min marta daqiqa tuzilishini kuzatish kuchini kengaytirdi" deb da'vo qilgan.[161] Rentgen diffraktsiyasi atomlar va ularning elektronlarning tarqalishini ko'rsatadigan atom darajasida o'lchamlari bo'lgan mikroskopga o'xshaydi.
Rentgen diffraktsiyasi, elektronlar va neytronlarning difraksiyasi moddalarning atom va molekulyar darajada, kristalli va kristall bo'lmagan tuzilishi haqida ma'lumot beradi. Bundan tashqari, ushbu usullar barcha materiallarning, noorganik, organik yoki biologik xususiyatlarini o'rganishda qo'llanilishi mumkin. Kristallarni difraksiyaviy tadqiq qilishning ahamiyati va qo'llanilishining xilma-xilligi tufayli ko'plab tadqiqotlar uchun Nobel mukofotlari berilgan.[162]
Giyohvand moddalarni aniqlash
Antibiotiklarni aniqlash uchun rentgen diffraktsiyasi ishlatilgan: sakkizta b-laktam (ampitsillin natriy, penitsillin G prokain, sefaleksin, ampitsillin trihidrat, benzatin penitsillin, natriy benzilpenitsillin, natriy sefotaksim, Natriy seftriakson ), uchta tetratsiklin (doksisiklin gidroxloridi, oksitratsiklin dehidrat, tetratsiklin gidroxloridi ) va ikkitasi makrolid (azitromitsin, eritromitsin estolat ) antibiotik preparatlari. Ushbu dorilarning har biri o'ziga xos rentgen difraksiyasi (XRD) sxemasiga ega, bu ularni aniqlashga imkon beradi.[163]
To'qimachilik tolalari va polimerlarining xarakteristikasi
Sud ekspertizasi har qanday iz dalillarga asoslanadi Lokardning almashinish printsipi. Bu "har qanday aloqa iz qoldiradi" deb ta'kidlaydi. Amalda, materialni o'tkazish amalga oshirilgan bo'lsa ham, uni aniqlash imkonsiz bo'lishi mumkin, chunki o'tkazilgan mablag 'juda oz.[164]
To'qimachilik tolalari - bu kristalli va amorf moddalar aralashmasi. Shuning uchun kristallik darajasini o'lchash rentgen diffraktometriyasi yordamida tolalarni tavsiflashda foydali ma'lumotlarni beradi. Xabarlarga ko'ra, stuldan topilgan "kristalli" konni aniqlash uchun rentgen diffraktsiyasi ishlatilgan. Depozit amorf ekanligi aniqlandi, ammo hozirgi diffraktsiya sxemasi polimetilmetakrilatnikiga to'g'ri keldi. Piroliz mass-spektrometriya Keyinchalik bu konni Boin kristalli parametrlarining polimetiltsianoakrilanasi sifatida aniqladi.[165]
Suyaklarni tekshirish
Suyaklarning isishi yoki yonishi suyak mineralida taniqli o'zgarishlarni keltirib chiqaradi, ular XRD texnikasi yordamida aniqlanishi mumkin. 500 ° C va undan yuqori haroratda dastlabki 15 daqiqada isitish jarayonida suyak kristallari o'zgarishni boshladi. Yuqori haroratlarda suyaklar kristallarining qalinligi va shakli barqarorlashgandek ko'rinadi, ammo namunalar pastroq haroratda yoki qisqa muddat qizdirilganda XRD izlari kristall parametrlarining keskin o'zgarishini ko'rsatdi.[166]
Integral mikrosxemalar
Murakkab tuzilishini tekshirish usuli sifatida rentgen difraksiyasi isbotlangan integral mikrosxemalar.[167]
Shuningdek qarang
- Beevers-Lipson strip
- Bragg difraksiyasi
- Kristallografik ma'lumotlar bazasi
- Kristallografik nuqta guruhlari
- Farq zichligi xaritasi
- Elektronlarning difraksiyasi
- Energiya dispersiv rentgen difraksiyasi
- Flack parametri
- Xenderson chegarasi
- Xalqaro kristallografiya yili
- Jon Desmond Bernal
- Ko'p zichlikdagi formalizm
- Neytron difraksiyasi
- Kukun difraksiyasi
- Pitografiya
- Sherrer tenglamasi
- Kichik burchakli rentgen nurlari (SAXS)
- Tuzilmani aniqlash
- Ultrafast rentgen
- Keng burchakli rentgen nurlari (WAXS)
Adabiyotlar
- ^ "Rezonansli rentgen nurlari | Shen laboratoriyasi". arpes.stanford.edu. Olingan 2019-07-10.
- ^ Kepler J (1611). Strena seu de Nive Sexangula. Frankfurt: G. Tampach. ISBN 3-321-00021-0.
- ^ Steno N (1669). Prodromus dissertationis da solido intra solidum naturaliter content. Florentsiya.
- ^ Gessel JFC (1831). Kristallometrie va Kristallonomie va Kristallographie. Leypsig.
- ^ Bravais A (1850). "Mémoire sur les systèmes formés par des points distribués regulièrement sur un plan ou dans l'espace". Journal de l'École Polytechnique. 19: 1.
- ^ Shafranovskiy I I & Belov N V (1962). Pol Evald (tahrir). "E. S. Fedorov" (PDF). 50 yil rentgen difraksiyasi. Springer: 351. ISBN 90-277-9029-9.
- ^ Schönflies A (1891). Kristallsysteme und Kristallstruktur. Leypsig.
- ^ Barlow V (1883). "Kristallarning ichki simmetriyasining ehtimoliy tabiati". Tabiat. 29 (738): 186. Bibcode:1883Natur..29..186B. doi:10.1038 / 029186a0. Shuningdek qarang Barlow, Uilyam (1883). "Kristallarning ichki simmetriyasining ehtimoliy tabiati". Tabiat. 29 (739): 205. Bibcode:1883Natur..29..205B. doi:10.1038 / 029205a0. Sohnke, L. (1884). "Kristallarning ichki simmetriyasining ehtimoliy tabiati". Tabiat. 29 (747): 383. Bibcode:1884Natur..29..383S. doi:10.1038 / 029383a0. S2CID 4072817. Barlow, VM. (1884). "Kristallarning ichki simmetriyasining ehtimoliy tabiati". Tabiat. 29 (748): 404. Bibcode:1884Natur..29..404B. doi:10.1038 / 029404b0. S2CID 4016086.
- ^ Eynshteyn A (1905). "Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt" [Nurni yaratish va o'zgartirishning evristik modeli]. Annalen der Physik (nemis tilida). 17 (6): 132. Bibcode:1905AnP ... 322..132E. doi:10.1002 / va.19053220607.. An Inglizcha tarjima dan foydalanish mumkin Vikipediya.
- ^ Taqqoslang: Eynshteyn A (1909). "Über die Entwicklung unserer Anschauungen über das Wesen und die Konststitution der Strahlung" [Radiatsiya tarkibi va mohiyatiga qarashlarimizning rivojlanishi]. Physikalische Zeitschrift (nemis tilida). 10: 817.. An Inglizcha tarjima dan foydalanish mumkin Vikipediya.
- ^ Pais A (1982). Nozik Rabbiy: Albert Eynshteynning ilmi va hayoti. Oksford universiteti matbuoti. ISBN 0-19-853907-X.
- ^ Kompton A (1923). "Yorug'lik elementlari tomonidan rentgen nurlarining tarqalishining kvant nazariyasi" (PDF). Fizika. Vah. 21 (5): 483. Bibcode:1923PhRv ... 21..483C. doi:10.1103 / PhysRev.21.483.
- ^ Bragg WH (1907). "Rengen nurlarining tabiati". Avstraliya Qirollik Ilmiy Jamiyatining operatsiyalari. 31: 94.
- ^ Bragg WH (1908). "G- va rentgen nurlarining tabiati". Tabiat. 77 (1995): 270. Bibcode:1908 yil Natur..77..270B. doi:10.1038 / 077270a0. S2CID 4020075. Shuningdek qarang Bragg, W. H. (1908). "Γ va rentgen nurlarining tabiati". Tabiat. 78 (2021): 271. Bibcode:1908 yil Natur..78..271B. doi:10.1038 / 078271a0. S2CID 4039315. Bragg, W. H. (1908). "Γ va rentgen nurlarining tabiati". Tabiat. 78 (2022): 293. Bibcode:1908 yil Natur..78..293B. doi:10.1038 / 078293d0. S2CID 3993814. Bragg, W. H. (1908). "Rentgen nurlarining tabiati". Tabiat. 78 (2035): 665. Bibcode:1908 yil Natur..78R.665B. doi:10.1038 / 078665b0. S2CID 4024851.
- ^ Bragg WH (1910). "Γ- va rentgen nurlari korpuskulyar gipotezasining oqibatlari va g-nurlari diapazoni". Fil. Mag. 20 (117): 385. doi:10.1080/14786441008636917.
- ^ Bragg WH (1912). "Ionizatsiyaning rentgen nurlari bilan bevosita yoki bilvosita tabiati to'g'risida". Fil. Mag. 23 (136): 647. doi:10.1080/14786440408637253.
- ^ a b Fridrix V; P ni qoqish; fon Laue M (1912). "Interferenz-Erscheinungen bei Röntgenstrahlen". Sitzungsberichte der Mathematisch-Physikalischen Classe der Königlich-Bayerischen Akademie der Wissenschaften zu Myunxen. 1912: 303.
- ^ fon Laue M (1914). "Rentgen aralashuvini aniqlash to'g'risida" (PDF). Nobel ma'ruzalari, fizika. 1901–1921. Olingan 2009-02-18.
- ^ Dana ES; Ford WE (1932). Mineralogiya darsligi (to'rtinchi nashr). Nyu-York: John Wiley & Sons. p. 28.
- ^ Andre Ginyer (1952). Rentgenografiya texnologiyasi. London: Hilger va Watts LTD. p. 271.
- ^ Bragg WL (1912). "X-nurlarining spekulyar refleksi". Tabiat. 90 (2250): 410. Bibcode:1912Natur..90..410B. doi:10.1038 / 090410b0. S2CID 3952319.
- ^ Bragg WL (1913). "Qisqa elektromagnit to'lqinlarning kristal bilan difraksiyasi". Kembrij falsafiy jamiyati materiallari. 17: 43.
- ^ Bragg (1914). "Die Reflexion der Röntgenstrahlen". Jahrbuch der Radioaktivität und Elektronik. 11: 350.
- ^ Bragg (1913). "Ba'zi kristallarning tuzilishi rentgen nurlari difraksiyasidan dalolat beradi". Proc. R. Soc. London. A89 (610): 248–277. Bibcode:1913RSPSA..89..248B. doi:10.1098 / rspa.1913.0083. JSTOR 93488.
- ^ Bragg WL; Jeyms RW; Bosanquet CH (1921). "Tosh-tuz tomonidan rentgen nurlarining qaytarilish intensivligi". Fil. Mag. 41 (243): 309. doi:10.1080/14786442108636225.
- ^ Bragg WL; Jeyms RW; Bosanquet CH (1921). "Tosh-tuz tomonidan rentgen nurlarining qaytarilish intensivligi. II qism". Fil. Mag. 42 (247): 1. doi:10.1080/14786442108633730.
- ^ Bragg WL; Jeyms RW; Bosanquet CH (1922). "Natriy va xlor atomlarida yadro atrofida elektronlarning tarqalishi". Fil. Mag. 44 (261): 433. doi:10.1080/14786440908565188.
- ^ a b Bragg WH; Bragg WL (1913). "Olmosning tuzilishi". Tabiat. 91 (2283): 557. Bibcode:1913 yil Natur..91..557B. doi:10.1038 / 091557a0. S2CID 3987932.
- ^ Bragg WH; Bragg WL (1913). "Olmosning tuzilishi". Proc. R. Soc. London. A89 (610): 277. Bibcode:1913RSPSA..89..277B. doi:10.1098 / rspa.1913.0084.
- ^ Bragg WL (1914). "Misning kristalli tuzilishi". Fil. Mag. 28 (165): 355. doi:10.1080/14786440908635219.
- ^ a b Bragg WL (1914). "X-ray spektrometri yordamida kristallarning tahlili". Proc. R. Soc. London. A89 (613): 468. Bibcode:1914RSPSA..89..468B. doi:10.1098 / rspa.1914.0015.
- ^ Bragg WH (1915). "Shpinel guruhi kristallari". Fil. Mag. 30 (176): 305. doi:10.1080/14786440808635400.
- ^ Nishikava S (1915). "Shpinel guruhining ba'zi kristallarining tuzilishi". Proc. Tokio matematikasi. Fizika. Soc. 8: 199.
- ^ Vegard L (1916). "Kristalli tahlil natijalari". Fil. Mag. 32 (187): 65. doi:10.1080/14786441608635544.
- ^ Aminoff G (1919). "Pirokroitning kristalli tuzilishi". Stokgolm Geol. Fören. Förh. 41: 407. doi:10.1080/11035891909447000.
- ^ Aminoff G (1921). "Über die Struktur des Magnesiumhydroxids". Z. Kristallogr. 56: 505.
- ^ Bragg WL (1920). "Sink oksidining kristalli tuzilishi". Fil. Mag. 39 (234): 647. doi:10.1080/14786440608636079.
- ^ Debije P; Sherrer P (1916). "Interferenz a regellos orientierten Teilchen im Röntgenlicht I". Physikalische Zeitschrift. 17: 277.
- ^ Fridrix V (1913). "Eine neue Interferenzerscheinung bei Röntgenstrahlen". Physikalische Zeitschrift. 14: 317.
- ^ Hull AW (1917). "X-ray kristalli analizining yangi usuli". Fizika. Vah. 10 (6): 661. Bibcode:1917PhRv ... 10..661H. doi:10.1103 / PhysRev.10.661.
- ^ Bernal JD (1924). "Grafitning tuzilishi". Proc. R. Soc. London. A106 (740): 749–773. JSTOR 94336.
- ^ Xassel O; Mack H (1924). "Über die Kristallstruktur des Graphits". Zeitschrift für Physik. 25 (1): 317. Bibcode:1924ZPhy ... 25..317H. doi:10.1007 / BF01327534. S2CID 121157442.
- ^ Hull AW (1917). "Temirning kristall tuzilishi". Fizika. Vah. 9 (1): 84. Bibcode:1917PhRv .... 9 ... 83.. doi:10.1103 / PhysRev.9.83.
- ^ Hull AW (1917 yil iyul). "Magniyning kristalli tuzilishi". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 3 (7): 470–3. Bibcode:1917PNAS .... 3..470H. doi:10.1073 / pnas.3.7.470. PMC 1091290. PMID 16576242.
- ^ a b "Atomlardan naqshlarga". Salomlar to'plami. Arxivlandi asl nusxasi 2013 yil 7 sentyabrda. Olingan 17 oktyabr 2013.
- ^ Wyckoff RWG; Posnjak E (1921). "Ammoniy xloroplatinatning kristalli tuzilishi". J. Am. Kimyoviy. Soc. 43 (11): 2292. doi:10.1021 / ja01444a002.
- ^ a b Bragg WH (1921). "Organik kristallarning tuzilishi". Proc. R. Soc. London. 34 (1): 33. Bibcode:1921PPSL ... 34 ... 33B. doi:10.1088/1478-7814/34/1/306.
- ^ Lonsdeyl K (1928). "Benzol halqasining tuzilishi". Tabiat. 122 (3082): 810. Bibcode:1928 yil natur.122..810L. doi:10.1038 / 122810c0. S2CID 4105837.
- ^ Poling L (1960). Kimyoviy bog'lanishning tabiati (3-nashr). Itaka, Nyu-York: Kornell universiteti matbuoti. ISBN 0-8014-0333-2.
- ^ Bragg WH (1922). "Antrasenning kristalli tuzilishi". Proc. R. Soc. London. 35 (1): 167. Bibcode:1922PPSL ... 35..167B. doi:10.1088/1478-7814/35/1/320.
- ^ Pauell HM; Evens RVG (1939). "Temir enneakarbonilning kristalli tuzilishi". J. Chem. Soc.: 286. doi:10.1039 / jr9390000286.
- ^ Bertran JA; Paxta; Dollase (1963). "CsReCl-dagi metall-metall bilan bog'langan, polinuklear kompleks anion4". J. Am. Kimyoviy. Soc. 85 (9): 1349. doi:10.1021 / ja00892a029.
- ^ Robinzon VT; Fergusson JE; Penfold BR (1963). "Anionni CsReCl-da konfiguratsiyasi4". London Kimyo Jamiyati Ma'lumotlari: 116.
- ^ Paxta FA, Curtis NF, Harris CB, Johnson BF, Lippard SJ, Mague JT va boshq. (1964 yil sentyabr). "Reniyning bir yadroli va polinukleer kimyosi (III): uning talaffuz qilingan gomofilligi". Ilm-fan. 145 (3638): 1305–7. Bibcode:1964Sci ... 145.1305C. doi:10.1126 / science.145.3638.1305. PMID 17802015. S2CID 29700317.
- ^ Paxta FA; Xarris (1965). "Dipotiy oktaxlorodirenat (III) dihidratning kristalli va molekulyar tuzilishi". Anorganik kimyo. 4 (3): 330. doi:10.1021 / ic50025a015.
- ^ Paxta FA (1965). "[Metall-Metal Bonding in [Re2X8]2− Ionlar va boshqa metall atom klasterlari ". Anorganik kimyo. 4 (3): 334. doi:10.1021 / ic50025a016.
- ^ Eberxardt VA; Kichik Krouford; Lipscomb WN (1954). "Bor gidridlarining valentlik tuzilishi". J. Chem. Fizika. 22 (6): 989. Bibcode:1954JChPh..22..989E. doi:10.1063/1.1740320.
- ^ Martin TW, Derewenda ZS (1999 yil may). "Ism - bog'lanish - H rishtasi". Tabiatning strukturaviy biologiyasi. 6 (5): 403–6. doi:10.1038/8195. PMID 10331860. S2CID 27195273.
- ^ Dunitz JD; Orgel LE; Boy A (1956). "Ferrosenning kristalli tuzilishi". Acta Crystallographica. 9 (4): 373. doi:10.1107 / S0365110X56001091.
- ^ Seiler P; Dunitz JD (1979). "Ferrosenning tartibsiz kristalli tuzilishini yangi talqini". Acta Crystallographica B. 35 (5): 1068. doi:10.1107 / S0567740879005598.
- ^ Wunderlich JA; Mellor DP (1954). "Zayza tuzining kristalli tuzilishi to'g'risida eslatma". Acta Crystallographica. 7: 130. doi:10.1107 / S0365110X5400028X.
- ^ Jarvis JAJ; Kilbourn BT; Owston PG (1970). "Zayza tuzining kristalli va molekulyar tuzilishini qayta aniqlash, KPtCl3.C2H4.H2O. tuzatish ". Acta Crystallographica B. 26 (6): 876. doi:10.1107 / S056774087000328X.
- ^ Jarvis JAJ; Kilbourn BT; Owston PG (1971). "Zayza tuzining kristalli va molekulyar tuzilishini qayta aniqlash, KPtCl3.C2H4.H2O ". Acta Crystallographica B. 27 (2): 366. doi:10.1107 / S0567740871002231.
- ^ Sevgi RA; Koetzle TF; Uilyams GJB; Andrews LC; Bau R (1975). "Zayza tuzi, KPtCl tuzilishini neytron difraksiyasi bilan o'rganish3(C2H4) .H2O ". Anorganik kimyo. 14 (11): 2653. doi:10.1021 / ic50153a012.
- ^ Xvecker, Maykl; Wrabetz, Sabine; Kruhnert, Jutta; Tsepei, Lenard-Istvan; Naumann d'Alnonkur, Raul; Kolen'Ko, Yuriy V.; Girgsdies, Frank; Shlyogl, Robert; Trunschke, Annette (2012). "Propanni akril kislotaga selektiv oksidlashda ish paytida faza toza M1 MoVTeNb oksidining sirt kimyosi". Kataliz jurnali. 285: 48–60. doi:10.1016 / j.jcat.2011.09.012. hdl:11858 / 00-001M-0000-0013-FB1F-C.
- ^ Mo va V asosli aralash oksid katalizatorlarida propan oksidlanishini kinetik tadqiqotlar. 2011.
- ^ Naumann d'Alnonkur, Raul; Tsepey, Lénard-Istvan; Xvecker, Maykl; Girgsdies, Frank; Shuster, Manfred E.; Shlyogl, Robert; Trunschke, Annette (2014). "Fazli sof MoVTeNb M1 oksidi katalizatorlari ustidan propan oksidlanishidagi reaktsiya tarmog'i". Kataliz jurnali. 311: 369–385. doi:10.1016 / j.jcat.2013.12.12.008. hdl:11858 / 00-001M-0000-0014-F436-1.
- ^ a b Braun, Dveyn (2012 yil 30 oktyabr). "NASA Rover-ning birinchi tuproq tadqiqotlari barmoq izi marslik minerallariga yordam beradi". NASA. Olingan 31 oktyabr, 2012.
- ^ Westgren A; Phragmén G (1925). "Cu-Zn, Ag-Zn va Au-Zn qotishmalarining rentgenologik tahlili". Fil. Mag. 50: 311. doi:10.1080/14786442508634742.
- ^ Bredli AJ; Thewlis J (1926). "B-guruch tuzilishi". Proc. R. Soc. London. 112 (762): 678. Bibcode:1926RSPSA.112..678B. doi:10.1098 / rspa.1926.0134.
- ^ Xyum-Roteri V (1926). "Intermetall birikmalarning tabiati, xususiyatlari va shakllanish shartlari bo'yicha tadqiqotlar (qalayning ba'zi birikmalariga alohida ishora bilan)". Metall instituti jurnali. 35: 295.
- ^ Bredli AJ; Gregori CH (1927). "Ba'zi uch qotishmalarning tuzilishi". Tabiat. 120 (3027): 678. Bibcode:1927 yil natur.120..678.. doi:10.1038 / 120678a0.
- ^ Westgren A (1932). "Zur Chemie der Legierungen". Angewandte Chemie. 45 (2): 33. doi:10.1002 / ange.19320450202.
- ^ Bernal JD (1935). "Metalllarning elektron nazariyasi". Kimyo taraqqiyoti to'g'risida yillik hisobotlar. 32: 181. doi:10.1039 / AR9353200181.
- ^ Poling L (1923). "Magniy Stannidning kristalli tuzilishi". J. Am. Kimyoviy. Soc. 45 (12): 2777. doi:10.1021 / ja01665a001.
- ^ Poling L (1929). "Murakkab ion ionlarining kristallarini aniqlash asoslari". J. Am. Kimyoviy. Soc. 51 (4): 1010. doi:10.1021 / ja01379a006.
- ^ Dikkinson RG; Raymond AL (1923). "Geksametilen-tetraminning kristalli tuzilishi" (PDF). J. Am. Kimyoviy. Soc. 45: 22. doi:10.1021 / ja01654a003.
- ^ Myuller A (1923). "Yog 'kislotalarini rentgen tekshiruvi". Kimyoviy jamiyat jurnali. 123: 2043. doi:10.1039 / ct9232302043.
- ^ Saville WB; Sheirer G (1925). "To'yingan alifatik ketonlarning rentgenologik tekshiruvi". Kimyoviy jamiyat jurnali. 127: 591. doi:10.1039 / ct9252700591.
- ^ Bragg WH (1925). "Yupqa plyonkalarni rentgen nurlari yordamida tekshirish". Tabiat. 115 (2886): 266. Bibcode:1925 yil Nat.115..266B. doi:10.1038 / 115266a0.
- ^ de Broyl M; Trillat JJ (1925). "Sur l'interprétation physique des specters X d'acides gras". Comptes rendus hebdomadaires des séances de l'Académie des fanlar. 180: 1485.
- ^ Trillat JJ (1926). "Rayons X et Composeés organiques à longe chaine. Spektrografiqlarni qayta tuzish bo'yicha tuzilmalar va leur yo'nalishlari". Annales de Physique. 6: 5. doi:10.1051 / anphys / 192610060005.
- ^ Caspari WA (1928). "Alifatik dikarboksilik kislotalarning kristallografiyasi". Kimyoviy jamiyat jurnali. ?: 3235. doi:10.1039 / jr9280003235.
- ^ Myuller A (1928). "Uzoq zanjirli birikmalarning rentgenologik tekshiruvi (uglevodorodlar).". Proc. R. Soc. London. 120 (785): 437. Bibcode:1928RSPSA.120..437M. doi:10.1098 / rspa.1928.0158.
- ^ Piper SH (1929). "Yog 'kislotalarining uzoq vaqt oralig'idan olinadigan ma'lumotlarning ayrim misollari". Faraday Jamiyatining operatsiyalari. 25: 348. doi:10.1039 / tf9292500348.
- ^ Myuller A (1929). "Uglevodorod zanjirining Zig-Zag tuzilishi bilan toq va juft raqamli zanjir aralashmalarining o'zgarishi o'rtasidagi bog'liqlik". Proc. R. Soc. London. 124 (794): 317. Bibcode:1929RSPSA.124..317M. doi:10.1098 / rspa.1929.0117.
- ^ Robertson JM (1936). "Fitalosiyaninlarni rentgenologik o'rganish, II qism". Kimyoviy jamiyat jurnali: 1195. doi:10.1039 / jr9360001195.
- ^ Crowfoot Hodgkin D (1935). "Insulinning rentgen nurli yagona kristalli fotosuratlari". Tabiat. 135 (3415): 591. Bibcode:1935 yil natur.135..591C. doi:10.1038 / 135591a0. S2CID 4121225.
- ^ Kendrew JK, Bodo G, Dintzis HM, Parrish RG, Vaykoff H, Fillips DC (1958 yil mart). "Miyoglobin molekulasining uch o'lchovli modeli rentgen analizida olingan". Tabiat. 181 (4610): 662–6. Bibcode:1958 yil natur.181..662K. doi:10.1038 / 181662a0. PMID 13517261. S2CID 4162786.
- ^ "Kimyo bo'yicha Nobel mukofoti 1962". www.nobelprize.org. Olingan 2018-01-31.
- ^ "PDB-da yozuvlar jadvali, eksperimental usul bilan tashkil etilgan".
- ^ "PDB statistikasi". RCSB Protein ma'lumotlar banki. Olingan 2010-02-09.
- ^ Scapin G (2006). "Strukturaviy biologiya va dori-darmonlarni kashf etish". Amaldagi farmatsevtika dizayni. 12 (17): 2087–97. doi:10.2174/138161206777585201. PMID 16796557.
- ^ Lundstrom K (2006 yil noyabr). "Membrana oqsillari uchun strukturaviy genomika". Uyali va molekulyar hayot haqidagi fanlar. 63 (22): 2597–607. doi:10.1007 / s00018-006-6252-y. PMID 17013556. S2CID 13432321.
- ^ Lundstrom K (2004 yil avgust). "Membrana oqsillari bo'yicha strukturaviy genomika: mini sharh". Kombinatorial kimyo va yuqori samaradorlikni skrining. 7 (5): 431–9. doi:10.2174/1386207043328634. PMID 15320710.
- ^ Chinte U, Shoh B, Chen YS, Pinkerton AA, Schall CA, Hanson BL (aprel 2007). "Kriyojenik (<20 K) geliyni sovutish oqsil kristallarining nurlanish zararlanishini yumshatadi". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 63 (Pt 4): 486-92. doi:10.1107 / s0907444907005264. PMID 17372353.
- ^ Kleydon, J .; Grivves, N. va Uorren, S. (2012). Organik kimyo (PDF) (2-nashr). Oksford universiteti matbuoti. p. 45. ISBN 978-0199270293.CS1 maint: bir nechta ism: mualliflar ro'yxati (havola)
- ^ Baskaran K, Duarte JM, Biyani N, Bliven S, Capitani G (oktyabr 2014). "PDB miqyosida evolyutsiyaga asoslangan oqsil-oqsil interfeyslarini baholash". BMC Strukturaviy Biologiya. 14 (1): 22. doi:10.1186 / s12900-014-0022-0. PMC 4274722. PMID 25326082.
- ^ Levy ED (noyabr 2007). "PiQSi: oqsilning to'rtinchi tuzilishini o'rganish". Tuzilishi. 15 (11): 1364–7. doi:10.1016 / j.str.2007.09.019. PMID 17997962.
- ^ Suryanarayana, C .; Norton, M. Grant (2013-06-29). X-ray difraksiyasi: amaliy yondashuv. Springer Science & Business Media. ISBN 9781489901484.
- ^ Greilinger, Alden B (1935). "Kristalli yo'nalishni aniqlash uchun orqa aks ettirish usuli". Zeitschrift für Kristallographie - Kristalli materiallar. 91 (1–6). doi:10.1524 / zkri.1935.91.1.424. S2CID 101434745.
- ^ Shunga o'xshash difraksiya naqshini lazer ko'rsatgichini a ga porlash orqali kuzatish mumkin ixcham disk yoki DVD; CD treklarining davriy oralig'i kristaldagi atomlarning davriy joylashuviga to'g'ri keladi.
- ^ "Morfologiya XRD tahlili | IMR TEST LABS". www.imrtest.com. Olingan 2018-04-30.
- ^ Jons N (2014 yil yanvar). "Kristallografiya: atom sirlari". Tabiat. 505 (7485): 602–3. Bibcode:2014 yil Natur.505..602J. doi:10.1038 / 505602a. PMID 24476871.
- ^ Miao, Tszianvey; Charalambous, Pambos; Kirz, Yanos; Sayre, Devid (1999). "Mikrometr o'lchamdagi kristal bo'lmagan namunalarni tasvirlash uchun rentgen kristallografiyasining metodologiyasini kengaytirish". Tabiat. 400 (6742): 342. Bibcode:1999 yil natur.400..342M. doi:10.1038/22498. S2CID 4327928.
- ^ Harp JM, Timm DE, Bunik GJ (1998 yil iyul). "Makromolekulyar kristalli tavlanish: kriyokristallografiya bilan bog'liq mozaikani engish". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 54 (Pt 4): 622-8. doi:10.1107 / S0907444997019008. PMID 9761858.
- ^ Harp JM, Hanson BL, Timm DE, Bunik GJ (1999 yil iyul). "Makromolekulyar kristalli tavlanish: texnikalar va o'zgaruvchilarni baholash". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 55 (Pt 7): 1329-34. doi:10.1107 / S0907444999005442. PMID 10393299.
- ^ Hanson BL, Harp JM, Bunik GJ (2003). "Yaxshi temperaturali oqsil kristali: tavlanuvchi makromolekulyar kristallar". Makromolekulyar kristallografiya, S qism. Enzimologiyadagi usullar. 368. 217-35 betlar. doi:10.1016 / S0076-6879 (03) 68012-2. ISBN 978-0-12-182271-2. PMID 14674276.
- ^ Geerlof A, Brown J, Coutard B, Egloff MP, Enguita FJ, Fogg MJ va boshq. (2006 yil oktyabr). "Tarkibiy proteomikada oqsil xarakteristikasining ta'siri". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 62 (Pt 10): 1125-36. doi:10.1107 / S0907444906030307. PMC 7161605. PMID 17001090.
- ^ Chernov AA (2003 yil aprel). "Protein kristallari va ularning o'sishi". Strukturaviy biologiya jurnali. 142 (1): 3–21. doi:10.1016 / S1047-8477 (03) 00034-0. PMID 12718915.
- ^ Terese Bergfors (2016). "Oqsillarni kristallashtirish bo'yicha qo'llanma".
- ^ Chayen, Naomi. "Yog 'ostida kristallanishning cheklovlari". Olingan 2 aprel 2019. Iqtibos jurnali talab qiladi
| jurnal =(Yordam bering) - ^ Rupp B, Vang J (2004 yil noyabr). "Protein kristallanishining bashoratli modellari". Usullari. 34 (3): 390–407. doi:10.1016 / j.ymeth.2004.03.031. PMID 15325656.
- ^ Chayen NE (2005 yil iyul). "Nukleatsiya va oqsil kristallanishidagi o'sishni ajratish usullari". Biofizika va molekulyar biologiyada taraqqiyot. 88 (3): 329–37. doi:10.1016 / j.pbiomolbio.2004.07.007. PMID 15652248.
- ^ Stock D, Perisic O, Löwe J (2005 yil iyul). "MRC molekulyar biologiya laboratoriyasida robotik nanolitr oqsil kristalizatsiyasi". Biofizika va molekulyar biologiyada taraqqiyot. 88 (3): 311–27. doi:10.1016 / j.pbiomolbio.2004.07.009. PMID 15652247.
- ^ Jeruzalmi D (2006). "Makromolekulyar kristallarning birinchi tahlili: biokimyo va rentgen difraksiyasi". Makromolekulyar kristallografiya protokollari, 2-jild. Molekulyar biologiya usullari. 364. 43-62 betlar. doi:10.1385/1-59745-266-1:43. ISBN 1-59745-266-1. PMID 17172760.
- ^ Helliwell JR (iyun 2005). "Protein kristalining mukammalligi va uni qo'llash". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 61 (Pt 6): 793-8. doi:10.1107 / S0907444905001368. PMID 15930642.
- ^ Vandenberg JM, Temkin H, Xamm RA, DiJuzeppe MA (1982). "InGaAsP / InP qatlamlaridagi qotishma oltin metallizatsiyasi kontaktlarini tizimli o'rganish". Amaliy fizika jurnali. 53 (11): 7385–7389. Bibcode:1982JAP .... 53.7385V. doi:10.1063/1.330364.
- ^ Vandenberg JM, Temkin H (1984). "InGaAsP / InP qatlamlari bilan oltin / to'siqsiz metallarning o'zaro ta'sirini in situ rentgenologik o'rganish". Amaliy fizika jurnali. 55 (10): 3676–3681. Bibcode:1984 JAP .... 55.3676V. doi:10.1063/1.332918.
- ^ Garman, E. F.; Shnayder, T. R. (1997). "Makromolekulyar kriyokristallografiya". Amaliy kristalografiya jurnali. 30 (3): 211. doi:10.1107 / S0021889897002677.
- ^ Schlichting I, Miao J (oktyabr 2012). "X-nurli elektronli lazerlar bilan strukturaviy biologiyada paydo bo'layotgan imkoniyatlar". Strukturaviy biologiyaning hozirgi fikri. 22 (5): 613–26. doi:10.1016 / j.sbi.2012.07.015. PMC 3495068. PMID 22922042.
- ^ Neutze R, Wouts R, van der Spoel D, Weckert E, Hajdu J (2000 yil avgust). "Femtosekundalik rentgen impulslari bilan biomolekulyar ko'rish imkoniyati". Tabiat. 406 (6797): 752–7. Bibcode:2000Natur.406..752N. doi:10.1038/35021099. PMID 10963603. S2CID 4300920.
- ^ Liu V, Vacker D, Gati C, Xan GW, Jeyms D, Vang D va boshq. (2013 yil dekabr). "G oqsillari bilan bog'langan retseptorlarning ketma-ket femtosekundlik kristallografiyasi". Ilm-fan. 342 (6165): 1521–4. Bibcode:2013 yil ... 342.1521L. doi:10.1126 / science.1244142. PMC 3902108. PMID 24357322.
- ^ Ravelli RB, Garman EF (2006 yil oktyabr). "Makromolekulyar kriyokristallografiyadagi radiatsiya shikastlanishi". Strukturaviy biologiyaning hozirgi fikri. 16 (5): 624–9. doi:10.1016 / j.sbi.2006.08.001. PMID 16938450.
- ^ Pauell HR (oktyabr 1999). "Rossmann Fourier MOSFLM-da avtomatik indekslash algoritmi". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 55 (Pt 10): 1690-5. doi:10.1107 / S0907444999009506. PMID 10531518.
- ^ Hauptman H (1997 yil oktyabr). "Protein kristallografiyasining bosqichma-bosqich usullari". Strukturaviy biologiyaning hozirgi fikri. 7 (5): 672–80. doi:10.1016 / S0959-440X (97) 80077-2. PMID 9345626.
- ^ Uson I, Sheldrick GM (oktyabr 1999). "Protein kristallografiyasining bevosita usullarining yutuqlari". Strukturaviy biologiyaning hozirgi fikri. 9 (5): 643–8. doi:10.1016 / S0959-440X (99) 00020-2. PMID 10508770.
- ^ a b Teylor G (2003 yil noyabr). "Faza muammosi". Acta Crystallographica. D bo'lim, Biologik kristallografiya. 59 (Pt 11): 1881-90. doi:10.1107 / S0907444903017815. PMID 14573942.
- ^ Ealick SE (oktyabr 2000). "Ko'p to'lqin uzunlikdagi anomal difraksion kristallografiyaning yutuqlari". Kimyoviy biologiyaning hozirgi fikri. 4 (5): 495–9. doi:10.1016 / S1367-5931 (00) 00122-8. PMID 11006535.
- ^ PDB faylidan 2NRL, qoldiqlar 17-32.
- ^ http://proteopedia.org/wiki/index.php/Garman_lab:_Interconversion_of_lysosomal_enzyme_specificities, olingan 28.11.2018.
- ^ Parkin G (1993). "O'tish metall komplekslarida bog'lanish-strech izomeriyasi: kristalografik ma'lumotlarning qayta baholanishi". Kimyoviy. Vah. 93 (3): 887–911. doi:10.1021 / cr00019a003.
- ^ Rietveld, H. M. (1969-06-02). "Yadro va magnit inshootlar uchun profilni takomillashtirish usuli". Amaliy kristalografiya jurnali. 2 (2): 65–71. doi:10.1107 / S0021889869006558.
- ^ Rietveld usuli. Yosh, R. A. (Robert Alan), 1921-. [Chester, Angliya]: Xalqaro Kristallogiya Ittifoqi. 1993 yil. ISBN 0198555776. OCLC 26299196.CS1 maint: boshqalar (havola)
- ^ "IUCr". www.iucr.org. Olingan 2019-04-06.
- ^ "Fullprof". www.ill.eu. Olingan 2019-04-06.
- ^ Petrichek, Vatslav; Dushek, Mixal; Palatinus, Lukash (2014-01-01). "Kristalografik hisoblash tizimi JANA2006: Umumiy xususiyatlar". Zeitschrift für Kristallographie - Kristalli materiallar. 229 (5). doi:10.1515 / zkri-2014-1737. ISSN 2196-7105. S2CID 101692863.
- ^ Lutterotti, Luka (2010 yil fevral). "Yupqa plyonkalarning difraksiyasida umumiy o'lcham - kuchlanish - stress - fakturani aniqlash uchun umumiy naqsh". Yadro asboblari va fizikani tadqiq qilish usullari B bo'lim: Materiallar va atomlar bilan nurlarning o'zaro ta'siri. 268 (3–4): 334–340. Bibcode:2010 NIMPB.268..334L. doi:10.1016 / j.nimb.2009.09.053. ISSN 0168-583X.
- ^ Lutterotti, L.; Bortolotti, M.; Iskiya, G.; Lonardelli, I .; Venk, H.-R. (2007), "Dietraktsiya tasvirlaridan Rietveld teksturasini tahlil qilish", O'ninchi Evropa changni difraksiyasi konferentsiyasi, OLDENBURG WISSENSCHAFTSVERLAG, doi:10.1524/9783486992540-020, ISBN 9783486992540
- ^ Lutterotti, L.; Matthies, S .; Venk, H.-R .; Shults, A. S.; Richardson, J. V. (1997-01-15). "Parchalangan vaqtdagi neytronlarning difraksiyasi spektrlaridan deformatsiyalangan ohaktoshning teksturasi va tuzilishini tahlil qilish". Amaliy fizika jurnali. 81 (2): 594–600. Bibcode:1997JAP .... 81..594L. doi:10.1063/1.364220. ISSN 0021-8979.
- ^ "RIETAN-FP-VENUS to'plami uchun tarqatiladigan fayllar". fujioizumi.verse.jp. Olingan 2019-04-06.
- ^ Tobi, Brayan X.; Von Dreele, Robert B. (2013-03-14). "GSAS-II: zamonaviy ochiq manbali barcha maqsadlarga mo'ljallangan kristallografiya dasturiy ta'minot to'plamining genezisi". Amaliy kristalografiya jurnali. 46 (2): 544–549. doi:10.1107 / s0021889813003531. ISSN 0021-8898.
- ^ "DIFFRAC.SUITE TOPAS - XRD dasturi, rentgen difraksiyasi". Bruker.com. Olingan 2019-04-06.
- ^ "Match! - Kukun difraksiyasidan fazalarni aniqlash". www.crystalimpact.com. Olingan 2019-04-06.
- ^ Montse-Kasas-Kabanas; Reynaud, dengiz piyodalari; Rikarte, Jokin; Horbax, Pavel; Rodriges-Karvaxal, Xuan (2016-12-01). "Xatolar: kengaytirilgan nuqsonli inshootlarni takomillashtirish dasturi". Amaliy kristalografiya jurnali. 49 (6): 2259–2269. doi:10.1107 / S1600576716014473. ISSN 1600-5767.
- ^ Patterson A. L. (1935). "Kristallardagi atomlararo masofalarning tarkibiy qismlarini aniqlashning bevosita usuli". Zeitschrift für Kristallographie. 90 (1–6): 517. doi:10.1524 / zkri.1935.90.1.517. S2CID 102041995.
- ^ "Fizika bo'yicha Nobel mukofoti 1914". Nobel jamg'armasi. Olingan 2008-10-09.
- ^ a b "Fizika bo'yicha Nobel mukofoti 1915". Nobel jamg'armasi. Olingan 2008-10-09.
- ^ a b "Kimyo bo'yicha Nobel mukofoti 1962". Nobelprize.org. Olingan 2008-10-06.
- ^ a b v "Fiziologiya yoki tibbiyot bo'yicha Nobel mukofoti 1962". Nobel jamg'armasi. Olingan 2007-07-28.
- ^ "Kimyo bo'yicha Nobel mukofoti 1964". Nobelprize.org. Olingan 2008-10-06.
- ^ a b "Kimyo bo'yicha Nobel mukofoti 1972". Nobelprize.org. Olingan 2008-10-06.
- ^ "Kimyo bo'yicha Nobel mukofoti 1976". Nobelprize.org. Olingan 2008-10-06.
- ^ a b "Kimyo bo'yicha Nobel mukofoti 1985". Nobelprize.org. Olingan 2008-10-06.
- ^ a b v "Kimyo bo'yicha Nobel mukofoti 1988". Nobelprize.org. Olingan 2008-10-06.
- ^ "Kimyo bo'yicha Nobel mukofoti 1997 yil". Nobelprize.org. Olingan 2008-10-06.
- ^ a b "Kimyo bo'yicha Nobel mukofoti 2003". Nobelprize.org. Olingan 2008-10-06.
- ^ "2006 yil kimyo bo'yicha Nobel mukofoti". Nobelprize.org. Olingan 2008-10-06.
- ^ a b v "Kimyo bo'yicha Nobel mukofoti 2009". Nobelprize.org. Olingan 2009-10-07.
- ^ "Kimyo bo'yicha Nobel mukofoti 2012". Nobelprize.org. Olingan 2012-10-13.
- ^ Maks fon Laue (1937). Laue diagrammalari. Bangalor Press. p. 9.
- ^ Frantsiya, André Authier, Institut de Minéralogie et de Physique des Milieux Condensés, Université P. et M. Curie, Parij (2013). Rentgenologik kristallografiyaning dastlabki kunlari (Birinchi nashr). Oksford: Oksford universiteti matbuoti. 1-8 betlar. ISBN 9780199659845.
- ^ Thangadurai S, Abraham JT, Srivastava AK, Moorth MN, Shukla SK, Anjaneyulu Y (iyul 2005). "Ba'zi beta-laktam, tetratsiklin va makrolidli antibiotik preparatlari uchun rentgen kukunlari difraksiyasi naqshlari". Analitik fanlar. 21 (7): 833–8. doi:10.2116 / analsci.21.833. PMID 16038505.
- ^ Rendle DF (2005 yil dekabr). "Sud tibbiyotida qo'llaniladigan kimyo yutuqlari". Kimyoviy jamiyat sharhlari. 34 (12): 1021–30. doi:10.1039 / b415890n. PMID 16284668.
- ^ Svarcova S, Kocí E, Bezdicka P, Hradil D, Hradilová J (sentyabr 2010). "Madaniy meros va sud ekspertizasi sohalarida qo'llaniladigan laboratoriya kukuni rentgen-mikro-difraksiyasini baholash". Analitik va bioanalitik kimyo. 398 (2): 1061–76. doi:10.1007 / s00216-010-3980-5. PMID 20640895. S2CID 11891108.
- ^ Hiller JC, Tompson TJ, Evison MP, Chamberlain AT, Wess TJ (dekabr 2003). "Eksperimental isitish paytida suyak minerallarining o'zgarishi: rentgen nurlari tarqalishini o'rganish". Biyomateriallar. 24 (28): 5091–7. doi:10.1016 / s0142-9612 (03) 00427-7. PMID 14568425.
- ^ Kortlend, Reychel (2017 yil 17 mart). "X-nurlari integral mikrosxemalarning 3D interyerini xaritada aks ettiradi". IEEE Spektri: Texnologiya, muhandislik va fan yangiliklari. Olingan 27 yanvar 2018.
Qo'shimcha o'qish
Kristallografiya bo'yicha xalqaro jadvallar
- Teo Xan, tahrir. (2002). Kristallografiya bo'yicha xalqaro jadvallar. A jild, kosmik guruh simmetriyasi (5-nashr). Dordrext: Kluwer Academic Publishers, uchun Xalqaro kristalografiya ittifoqi. ISBN 0-7923-6590-9.
- Maykl G. Rossmann; Eddi Arnold, tahrir. (2001). Kristallografiya bo'yicha xalqaro jadvallar. F jild, biologik molekulalarning kristalografiyasi. Dordrext: Kluwer Academic Publishers, Xalqaro Kristallografiya Ittifoqi uchun. ISBN 0-7923-6857-6.
- Teo Xan, tahrir. (1996). Kristallografiya bo'yicha xalqaro jadvallar. A hajmining qisqacha o'qitish nashri, kosmik guruh simmetriyasi (4-nashr). Dordrext: Kristallografiya xalqaro ittifoqi uchun Kluwer Academic Publishers. ISBN 0-7923-4252-6.
Maqolalar to'plamlari
- Charlz V. Karter; Robert M. Shirin., Tahrir. (1997). Makromolekulyar kristallografiya, A qism (Enzimologiya metodlari, 276-oyat). San-Diego: Akademik matbuot. ISBN 0-12-182177-3.
- Kichik Charlz V. Karter; Robert M. Shirin., Tahrir. (1997). Makromolekulyar kristallografiya, B qism (Enzimologiya metodlari, 277-oyat). San-Diego: Akademik matbuot. ISBN 0-12-182178-1.
- A. Dyukruix; R. Giege, nashrlar. (1999). Nuklein kislotalari va oqsillarni kristallashtirish: amaliy yondashuv (2-nashr). Oksford: Oksford universiteti matbuoti. ISBN 0-19-963678-8.
Darsliklar
- Birkholz M, Fewster PF va Genzel C (2005) hissalari bilan. "1-bob: rentgen-difraksiyaning printsiplari". X-Ray Scattering tomonidan ingichka kino tahlili. Vaynxaym: Vili-VCH. ISBN 978-3-527-31052-4.
- Blow D (2002). Biologlar uchun kristallografiya sxemasi. Oksford: Oksford universiteti matbuoti. ISBN 0-19-851051-9.
- Berns G.; Glazer A M (1990). Olimlar va muhandislar uchun kosmik guruhlar (2-nashr). Boston: Academic Press, Inc. ISBN 0-12-145761-3.
- Klegg V (1998). Kristall tuzilishini aniqlash (Oksford kimyo astarlari). Oksford: Oksford universiteti matbuoti. ISBN 0-19-855901-1.
- Cullity B.D. (1978). Rentgen difraksiyasining elementlari (2-nashr). Reading, Massachusets: Addison-Uesli nashriyot kompaniyasi. ISBN 0-534-55396-6.
- Drenth J (1999). Proteinli rentgen kristalografiyasining asoslari. Nyu-York: Springer-Verlag. ISBN 0-387-98587-5.
- Giacovazzo C (1992). Kristallografiya asoslari. Oksford: Oksford universiteti matbuoti. ISBN 0-19-855578-4.
- Glusker JP; Lyuis M; Rossi M (1994). Kimyogarlar va biologlar uchun kristall tuzilishini tahlil qilish. Nyu-York: VCH nashriyotlari. ISBN 0-471-18543-4.
- Massa V (2004). Kristal tuzilishini aniqlash. Berlin: Springer. ISBN 3-540-20644-2.
- McPherson A (1999). Biologik makromolekulalarning kristallanishi. Cold Spring Harbor, NY: Cold Spring Harbor laboratoriyasining matbuoti. ISBN 0-87969-617-6.
- McPherson A (2003). Makromolekulyar kristallografiyaga kirish. John Wiley & Sons. ISBN 0-471-25122-4.
- McRee DE (1993). Amaliy oqsil kristalografiyasi. San-Diego: Akademik matbuot. ISBN 0-12-486050-8.
- O'Keeff M; Hyde B G (1996). Kristalli inshootlar; I. Naqshlar va simmetriya. Vashington, DC: Amerika mineralogik jamiyati, Monografiyalar seriyasi. ISBN 0-939950-40-5.
- Rodos G (2000). Kristallografiya kristalli tiniq qildi. San-Diego: Akademik matbuot. ISBN 0-12-587072-8., Tanlangan boblarning PDF nusxasi
- Rupp B (2009). Biyomolekulyar kristallografiya: printsiplari, amaliyoti va strukturaviy biologiyada qo'llanilishi. Nyu-York: Garland fani. ISBN 978-0-8153-4081-2.
- Uorren BE (1969). Rentgen difraksiyasi. Nyu York. ISBN 0-486-66317-5.
- Zachariasen WH (1945). Kristallarda rentgen difraksiyasi nazariyasi. Nyu-York: Dover nashrlari. LCCN 67026967.
Amaliy hisoblash ma'lumotlarini tahlil qilish
- Young, R.A., ed. (1993). Rietveld usuli. Oksford: Oksford universiteti matbuoti va xalqaro kristalografiya ittifoqi. ISBN 0-19-855577-6.
Tarixiy
- Bijvoet JM; Burgers WG; Hägg G; eds. (1969). X-nurlarini kristallar difraksiyasi to'g'risida dastlabki hujjatlar. Men. Utrext: A. Oosthoekning Uitgeversmaatschappij N.V. tomonidan nashr etilgan Xalqaro Kristalografiya Ittifoqi uchun.CS1 maint: qo'shimcha matn: mualliflar ro'yxati (havola)
- Bijvoet JM; Burgers WG; Hägg G, tahrir. (1972). X-nurlarini kristallar difraksiyasi to'g'risida dastlabki hujjatlar. II. Utrext: A. Oosthoekning Uitgeversmaatschappij N.V. tomonidan nashr etilgan Xalqaro Kristalografiya Ittifoqi uchun.
- Bragg G L; Phillips D C & Lipson H (1992). Rentgen-analizning rivojlanishi. Nyu-York: Dover. ISBN 0-486-67316-2.
- Evald, PP; va ko'plab kristalograflar, nashrlar. (1962). Ellik yillik rentgen difraksiyasi. Utrext: A. Oosthoekning Uitgeversmaatschappij N.V. tomonidan nashr etilgan Xalqaro Kristalografiya Ittifoqi uchun. doi:10.1007/978-1-4615-9961-6. ISBN 978-1-4615-9963-0.
- Evald, P. P., muharrir 50 yil rentgen difraksiyasi (IUCr XVIII Kongress, Glazgo, Shotlandiya, Kristallografiya Xalqaro Ittifoqi uchun pdf formatida qayta nashr etilgan).
- Fridrix V (1922). "Die Geschichte der Auffindung der Röntgenstrahlinterferenzen". Naturwissenschaften vafot etdi. 10 (16): 363. Bibcode:1922NW ..... 10..363F. doi:10.1007 / BF01565289. S2CID 28141506.
- Lonsdeyl, K (1949). Kristallar va rentgen nurlari. Nyu-York: D. van Nostran.
- "Hayotning tuzilmalari". AQSh Sog'liqni saqlash va aholiga xizmat ko'rsatish vazirligi. 2007 yil. Iqtibos jurnali talab qiladi
| jurnal =(Yordam bering)
Tashqi havolalar
| Kutubxona resurslari haqida Rentgenologik kristallografiya |
O'quv qo'llanmalari
- Kristallografiyani o'rganish
- Oddiy, texnik bo'lmagan kirish
- Kristallografiya to'plami dan., video seriyalar Qirollik instituti
- "Kichik molekulalarni kristallashtirish" (PDF ) da Illinoys Texnologiya Instituti veb-sayt
- Xalqaro kristalografiya ittifoqi
- Kristallografiya 101
- Interaktiv tuzilish omillari bo'yicha qo'llanma, 2D kristalning difraktsiya naqshining xususiyatlarini namoyish etadi.
- Furye transformatsiyasining rasm kitobi, 2D dagi kristall va difraksiya naqshlari o'rtasidagi munosabatni aks ettiradi.
- X-ray kristallografiyasi va tuzilishini aniqlash bo'yicha ma'ruza matnlari
- Nan o'lchovli materiallarni tahlil qilishning zamonaviy rentgen sochish usullari to'g'risida onlayn ma'ruza Richard J. Matyi tomonidan
- Interaktiv kristalografiya xronologiyasi dan Qirollik instituti
Birlamchi ma'lumotlar bazalari
- Kristallografiya ochiq ma'lumotlar bazasi (COD)
- Protein ma'lumotlar banki (PDB )
- Nuklein kislotasi to'g'risidagi ma'lumotlar banki (NDB)
- Kembrijning tarkibiy ma'lumotlar bazasi (CSD )
- Anorganik kristalli tuzilish ma'lumotlar bazasi (ICSD )
- Biologik makromolekulalarning kristallanish bazasi (BMCD)
Derivativ ma'lumotlar bazalari
- PDBsum
- Proteopediya - oqsillar va boshqa molekulalarning hamkorlikdagi, 3 o'lchamli ensiklopediyasi
- RNABase
- PDB ligandlarining HIC-Up ma'lumotlar bazasi
- Oqsillarning strukturaviy tasnifi ma'lumotlar bazasi
- CATH oqsillari tuzilishi tasnifi
- 3D tuzilishi ma'lum bo'lgan transmembran oqsillari ro'yxati
- Membranlar ma'lumotlar bazasidagi oqsillarning yo'nalishlari
Strukturaviy tekshirish
- MolProbity tarkibiy tasdiqlash to'plami
- ProSA-veb
- NQ-Flipper (Asn va Gln qoldiqlarining noqulay rotamerlarini tekshiring)
- DALI-server (berilgan oqsilga o'xshash oqsillarni aniqlaydi)






















