Depressiya biologiyasi - Biology of depression
Ilmiy tadqiqotlar shuni ko'rsatdiki, turli xil miya sohalari odamlarda o'zgaruvchan faollikni namoyish etadi katta depressiv buzilish, va bu psixologik yoki situatsion sabablarni ta'kidlaydigan nazariyalardan farqli o'laroq, kasallikning biokimyoviy kelib chiqishini aniqlashga intilayotgan turli xil nazariyalar tarafdorlarini rag'batlantirdi. Ushbu qo'zg'atuvchi guruhlarni qamrab oluvchi omillarga oziqlanish etishmovchiligi kiradi magniy, D vitamini va triptofan situatsion kelib chiqishi bilan, ammo biologik ta'sirga ega. Ga oid bir nechta nazariyalar depressiyaning biologik asosli sababi yillar davomida taklif qilingan, shu jumladan atrofida aylanib yurgan nazariyalar monoamin nörotransmitterlari, neyroplastiklik, neyrogenez, yallig'lanish va sirkadiyalik ritm. Jismoniy kasalliklar, shu jumladan hipotiroidizm va mitoxondriyal kasallik, shuningdek, depressiv simptomlarni keltirib chiqarishi mumkin.[1][2]
Nerv davrlari ruhiy tushkunlik bilan bog'liq bo'lgan hissiyotlarni yaratish va tartibga solish bilan shug'ullanadiganlar, shuningdek mukofotlash bilan bog'liq. Odatda anormalliklar lateral prefrontal korteksda uchraydi, ularning taxminiy funktsiyasi odatda hissiyotlarni tartibga solishni o'z ichiga oladi. Kabi hissiyot va mukofotni yaratish bilan bog'liq mintaqalar amigdala, oldingi singulat korteksi (ACC), orbitofrontal korteks (OFC) va striatum tez-tez ham aloqador. Ushbu mintaqalar monoaminerjik yadrolar tomonidan innervatsiya qilingan va taxminiy dalillar g'ayritabiiy holat uchun potentsial rolni ko'rsatmoqda monoaminerjik faoliyat.[3][4]
Genetik omillar
Genlarni o'rganish qiyinligi
Tarixiy jihatdan, nomzod genlarni o'rganish tadqiqotlarning asosiy yo'nalishi bo'lgan. Ammo, genlar soni to'g'ri nomzod genini tanlash ehtimolini kamaytirar ekan, I turidagi xatolar (noto'g'ri ijobiy) ehtimol katta. Nomzodlarning genlari bo'yicha tadqiqotlar tez-tez bir qator kamchiliklarga ega, shu jumladan tez-tez genotiplashda xatolar va statistik jihatdan zaif. Ushbu ta'sirlar genlarni genlarning o'zaro ta'sirini hisobga olmasdan odatiy baholash bilan murakkablashadi. Ushbu cheklovlar biron bir nomzod geni genomen ahamiyatga ega bo'lmagani bilan aks etadi.[5]
Gen nomzodlari
5-HTTLPR
2003 yildagi bir tadqiqot shuni ko'rsatdiki, a gen-muhitning o'zaro ta'siri (GxE) nima uchun hayotiy stress ba'zi odamlarda depressiv epizodlar uchun bashorat qiluvchi omil ekanligini tushuntirishi mumkin, ammo boshqalarda serotonin-transportyor bilan bog'langan promotor mintaqaning allelik o'zgarishiga qarab (5-HTTLPR ).[6] 2019 yildan boshlab 5-HTTLPR GxE o'zaro ta'sirining oltita meta-tahlillari o'tkazildi. 2009 yilgi ikkita meta-tahlil null topilmalar haqida xabar berdi,[7][8] ko'proq liberal qo'shilish mezonlari bilan 2011 meta-tahlilida muhim munosabatlar haqida xabar berilgan.[9] 2016 yilgi meta-tahlil GxE o'zaro ta'sirining dalillari eng yaxshi holatda zaif degan xulosaga keldi.[10] 2018 meta-tahlilida zaif, ammo sezilarli heterojenlik bilan cheklangan muhim munosabatlar haqida xabar berilgan.[11] 2019 meta-tahlilida zo'ravonlik bilan o'z joniga qasd qilish xatti-harakatlari (MDDning asoratlari) va gen o'rtasidagi munosabatlar haqida xabar berilgan.[12]
BDNF
BDNF polimorfizmlar ham genetik ta'sirga ega deb faraz qilingan, ammo replikatsiya natijalari aralashgan va 2005 yildan boshlab meta-tahlil uchun etarli bo'lmagan.[13] Tadqiqotlar, shuningdek, o'z joniga qasd qilish harakati bilan BDNF ishlab chiqarishining pasayishi bilan bog'liqligini ko'rsatadi.[14] Biroq, gen va atrof-muhitning o'zaro ta'sirini o'rganish natijalari shuni ko'rsatadiki, hozirgi BDNF depressiya modellari juda sodda.[15] 2008 yilgi tadqiqotlar o'zaro ta'sirlarni (biologik) topdi epistaz ) BDNF signalizatsiya yo'llarida va serotonin tashuvchisi; BDNF Val66Met serotoninga nisbatan sezgirlikni pasaytirishi taxmin qilingan allelning qisqa 5-HTTLPR alleli bo'lgan odamlarda himoya ta'sirini ko'rsatishi aniqlandi, aks holda stressli hodisalardan keyin odamlarni depressiv epizodlarga moyil qiladi.[16] Shunday qilib, stress va antidepressantlarga neyroplastik ta'sir ko'rsatadigan BDNF vositachiligidagi signalizatsiya boshqa genetik va atrof-muhit modifikatorlari ta'sirida.[15]
SIRT1 va LHPP
2015 yilda xan xitoylik ayollarda o'tkazilgan GWAS tadqiqotlari yaqin atrofdagi intronik mintaqalarda ikkita variantni ijobiy aniqladi SIRT1 va LHPP genom bo'yicha muhim birlashma bilan.[17][18]
Norepinefrin tashuvchisi polimorfizmlari
Norepinefrin tashuvchisi polimorfizmlari va tushkunlik o'rtasidagi o'zaro bog'liqlikni topishga urinishlar salbiy natijalarni berdi.[19]
Bitta ko'rib chiqishda bir nechta tez-tez o'rganib chiqilgan nomzodlarning genlari aniqlandi. Uchun kodlovchi genlar 5-HTT va 5-HT2A retseptorlari depressiya va davolanishga javob bilan mos kelmagan. Aralash natijalar topildi miyadan kelib chiqadigan neyrotrofik omil (BDNF) Val66Met polimorfizmlari. Polimorfizmlar triptofan gidroksilaza gen o'z joniga qasd qilish harakati bilan taxminiy ravishda bog'liqligi aniqlandi.[20] 2008 yilda nashr etilgan 182 ta holat bo'yicha boshqariladigan genetik tadqiqotlarning meta-tahlillari topildi Apolipoprotein E verepsilon 2 himoya bo'lishi kerak va xavf tug'diradigan GNB3 825T, MTHFR 677T, SLC6A4 44bp kiritish yoki o'chirish va SLC6A3 40 bpVNTR 9/10 genotipi.[21]
Sirkadiyalik ritm

Uyqu
Depressiya anormallik bilan bog'liq bo'lishi mumkin sirkadiyalik ritm,[22] yoki biologik soat. Masalan, tez ko'z harakati (REM) uxlash - bu bosqich orzu qilish sodir bo'ladi - tushkunlikka tushgan odamlarga tezda etib borishi va kuchli bo'lishi mumkin. REM uyqusi pasayishiga bog'liq serotonin darajalari miya sopi,[23] va antidepressantlar singari miya sopi tuzilishida serotonerjik ohangni kuchaytiradigan birikmalar tomonidan buziladi.[23] Umuman olganda, serotonerjik tizim uxlash vaqtida eng kam faol va bedorlikda eng faol bo'ladi. Tufayli uzoq vaqt bedorlik uyqusizlik[22] serotonerjik neyronlarni faollashtiradi, bu antidepressantlarning terapevtik ta'siriga o'xshash jarayonlarga olib keladi, masalan, selektiv serotoninni qaytarib olish inhibitörleri (SSRI). Tushkunlikka tushgan shaxslar bir kecha uyqusiz qolgandan keyin kayfiyatni sezilarli darajada ko'tarishi mumkin. SSRI to'g'ridan-to'g'ri terapevtik ta'siri uchun markaziy serotonerjik nörotransmisyonning ko'payishiga bog'liq bo'lishi mumkin, xuddi shu tizim uyqu va bedorlik tsikllariga ta'sir qiladi.[23]
Nur terapiyasi
Ta'siri bo'yicha tadqiqotlar nur terapiyasi kuni mavsumiy affektiv buzilish yorug'lik etishmovchiligi serotonerjik tizimdagi faollikning pasayishi va uyqu tsiklidagi anormalliklar, xususan uyqusizlik bilan bog'liqligini ko'rsatadi. Yorug'likka ta'sir qilish serotonerjik tizimga ham qaratilgan bo'lib, bu tizim depressiyada muhim rol o'ynashi uchun ko'proq yordam beradi.[24] Uyqusiz qolish va nur terapiyasi ikkalasi ham antidepressant dorilar bilan bir xil miya nörotransmitter tizimi va miya sohalarini maqsad qilib qo'ygan va hozirgi kunda depressiyani davolash uchun klinik jihatdan foydalanilmoqda.[25] MDD kasalxonasiga yotqizilgan odamlarda chuqur tushkunlikni to'xtatish uchun yorug'lik terapiyasi, uyqusizlik va uxlash vaqtini almashtirish (uyqu fazasini oldindan davolash) tezkor ravishda birgalikda qo'llaniladi.[24]
Uyquning ko'payishi va kamayishi depressiya uchun xavf omilidir.[26] MDD bilan kasallangan odamlar ba'zida mavsumiy bo'lmagan depressiyada ham simptomlarning zo'ravonligining kunduzgi va mavsumiy o'zgarishini ko'rsatadilar. Kundalik kayfiyatni yaxshilash dorsal asab tarmoqlari faoliyati bilan bog'liq edi. O'rtacha yadro haroratining oshishi ham kuzatildi. Gipotezalardan birida depressiya o'zgarishlar siljishi natijasida yuzaga kelgan degan taxminlar mavjud.[27]
Kunduzgi yorug'lik ta'sir qilish ba'zi depressiyalarning mavsumiyligi asosida bo'lishi mumkin bo'lgan serotonin tashuvchisi faolligining pasayishi bilan bog'liq.[28]
Monoaminlar
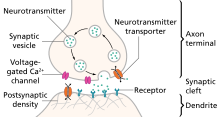
Monoaminlar bor neyrotransmitterlar shu jumladan serotonin, dopamin, noradrenalin va epinefrin.[29]
Depressiyaning monoamin gipotezasi
Ko'pchilik antidepressant dorilar keskin ko'payadi sinaptik monoamin nörotransmitteri, serotonin darajasi, ammo ular boshqa ikkita neyrotransmitter, noradrenalin va dofamin darajasini ham oshirishi mumkin. Ushbu samaradorlikni kuzatish depressiyaning monoamin gipotezasi, bu ma'lum nörotransmitterlarning etishmasligi depressiya uchun javobgardir va hatto ba'zi nörotransmitterlarning o'ziga xos alomatlar bilan bog'liqligini ta'kidlaydi. Oddiy serotonin darajasi kayfiyat va xulq-atvorni tartibga solish, uxlash va ovqat hazm qilish bilan bog'liq; norepinefrinni jangga yoki parvozga javob; dopamin harakatga, zavq va motivatsiyaga ta'sir qiladi. Ba'zilar, monoaminlar va fenotiplar, masalan, uyqudagi serotonin va o'z joniga qasd qilish, disforiyada norepinefrin, charchoq, befarqlik, kognitiv funktsiya buzilishi va motivatsiya va psixomotor alomatlarning yo'qolishida dofamin kabi munosabatlarni taklif qildilar.[30] Depressiyaning monoamin gipotezasining asosiy cheklovi antidepressant bilan davolashni boshlash va simptomlarning yaxshilanishi o'rtasidagi terapevtik kechikishdir. Ushbu terapevtik kechikishning bir izohi shundaki, sinaptik serotoninning ko'payishi vaqtinchalik, chunki serotonerjik neyronlarning otilishi dorsal rap 5-HT faolligi orqali moslashish1A autoreseptorlar. Antidepressantlarning terapevtik ta'siri ma'lum vaqt davomida autoreseptor desensitizatsiyasidan kelib chiqadi va natijada serotonerjik neyronlarning otilishini kuchaytiradi.[31]

Serotonin
Depressiyadagi serotoninning dastlabki tadqiqotlari serotonin metaboliti kabi periferik choralarni o'rganib chiqdi 5-gidroksiindoleasetik kislota (5-HIAA) va trombotsitlarni bog'lash. Natijalar umuman nomuvofiq bo'lgan va markaziy asab tizimiga umuman mos kelmasligi mumkin. Ammo dalillar retseptorlarni bog'lash tadqiqotlar va farmakologik muammolar depressiyada serotonin nörotransmisyonunun buzilishi uchun ba'zi dalillarni keltirib chiqaradi.[32] Serotonin bilvosita kayfiyatni o'zgartirish orqali ta'sir qilishi mumkin hissiy ishlov berish tarafkashliklari ikkala kognitiv / xulq-atvor va asab darajasida ko'rinadi.[33][32] Farmakologik jihatdan kamaytiradigan serotonin sintezi va farmakologik jihatdan kuchaytiradigan sinaptik serotonin, o'z navbatida, salbiy ta'sirchan tomonlarni keltirib chiqarishi va susaytirishi mumkin. Ushbu emotsional ishlov berish tarafkashliklari terapevtik bo'shliqni tushuntirishi mumkin.[33]
Dopamin
Dopaminerjik tizimlarda turli xil anormalliklar kuzatilgan bo'lsa-da, natijalar bir-biriga mos kelmaydi. MDD bilan kasallangan odamlar uchun mukofotning ko'payishi dekstroamfetamin nazorati bilan taqqoslaganda va bu tabiiy gipoaktivlik tufayli dopaminerjik yo'llarning yuqori sezuvchanligidan kelib chiqadi degan fikrlar mavjud. D4 va D3 retseptorlari polimorfizmlari depressiyaga ta'sir qilgan bo'lsa-da, assotsiatsiyalar doimiy ravishda takrorlanmagan. Shu kabi nomuvofiqlik o'limdan keyingi tadqiqotlarda aniqlandi, ammo turli xil dopamin retseptorlari agonistlari MDDni davolashda umid baxsh etadi.[34] Kamayganligi haqida ba'zi dalillar mavjud nigrostriatal yo'l melankolik depressiyali odamlarda faollik (psixomotor sustkashlik).[35] Dopaminning ruhiy tushkunlikdagi rolini yanada qo'llab-quvvatlash - bu kamaytirilgan miya omurilik suyuqligi va dopaminning bo'yin metabolitlarini izchil topish,[36] shuningdek, o'zgartirilgan o'limdan keyingi topilmalar Dopamin retseptorlari D3 va dopamin tashuvchisi ifoda.[37] O'qish kemiruvchilar dopaminerjik tizimlarning stress bilan bog'liq disfunktsiyasini o'z ichiga olgan potentsial mexanizmni qo'llab-quvvatladilar.[38]
Katekolaminlar
Depressiyada adrenergik faollik pasayganligini ko'rsatuvchi bir qator dalillar keltirilgan. Topilmalar orasida tirozin gidroksilaza faolligining pasayishi, lokus koerulasi hajmining pasayishi va ortishi bor alfa 2 adrenergik retseptorlari zichligi va kamayganligi alfa 1 adrenergik retseptorlari zichlik.[36] Bundan tashqari, sichqonlar modellarida noradrenali transporterni nokaut qilish ularning stressga chidamliligini oshiradi, depressiyada norepinefrinni keltirib chiqaradi.[39]
Monoaminlarning rolini o'rganish uchun foydalaniladigan usullardan biri bu monoaminni yo'q qilishdir. Tugash triptofan (serotoninning kashshofi), tirozin va fenilalanin (dopaminning prekursorlari) depressiyaga moyil bo'lganlarning kayfiyatini pasayishiga olib keladi, ammo moyilligi yo'q odamlarda emas. Boshqa tomondan, dopamin va norepinefrin sintezining inhibatsiyasi alfa-metil-para-tirozin doimiy ravishda kayfiyatning pasayishiga olib kelmaydi.[40]
Monoamin oksidaz
Monoamin gipotezasining kelib chiqishi shundan dalolat beradi monoamin oksidaza A Monoaminlarni almashinadigan ferment (MAO-A) depressiv odamlarda haddan tashqari faol bo'lishi mumkin. Bu, o'z navbatida, monoaminlarning pasayishiga olib keladi. Ushbu gipoteza a tomonidan qo'llab-quvvatlandi UY HAYVONI ba'zi bir tushkunlikka tushgan odamlarning miyasida MAO-A faolligining sezilarli darajada ko'tarilganligini aniqlagan tadqiqot.[41] Genetik tadqiqotlarda MAO-A bilan bog'liq genlarning o'zgarishi doimiy ravishda depressiya bilan bog'liq emas.[42][43] Monoamin gipotezasining taxminlaridan farqli o'laroq, MAO-A ning pasaytirilgan, ammo kuchaymagan faolligi o'spirinlarda depressiv alomatlar bilan bog'liq edi. Ushbu assotsiatsiya nafaqat yomon muomalada bo'lgan yoshlarda kuzatilgan, bu ham biologik (MAO genlari), ham psixologik (yomon munosabatda bo'lish) omillari depressiv kasalliklarni rivojlanishida muhim ahamiyatga ega ekanligini ko'rsatdi.[44] Bundan tashqari, ba'zi bir dalillar shuni ko'rsatadiki, kimyoviy muvozanatning o'zgarishi emas, balki neyron tarmoqlarida axborotni qayta ishlashni buzilishi depressiyaga olib kelishi mumkin.[45]
Cheklovlar
1990-yillardan boshlab, tadqiqotlar monoamin gipotezasining ko'plab cheklovlarini aniqladi va uning nomuvofiqligi psixiatriya jamoasida tanqid qilindi.[46] Birinchidan, serotonin tizimining buzilishi depressiyaning yagona sababi bo'lishi mumkin emas. Barcha davolangan bemorlar emas antidepressantlar odatda sinaptik serotoninning tez o'sishiga qaramay yaxshilanishlarni ko'rsating. Agar kayfiyatda sezilarli yaxshilanishlar yuz bersa, bu kamida ikki yoki to'rt hafta davomida bo'lmaydi. Ushbu kechikishning mumkin bo'lgan izohlaridan biri shundaki, nörotransmitter faolligini oshirish bir necha hafta davom etishi mumkin bo'lgan retseptorlarning desensitizatsiyasining natijasidir.[47] Intensiv tekshiruv MDD bilan kasallangan odamlarda ma'lum bir monoamin tizimining asosiy disfunktsiyasining ishonchli dalillarini topa olmadi. Kabi monoamin tizimi orqali harakat qilmaydigan antidepressantlar tianeptin va opipramol, azaldan ma'lum bo'lgan. Shuningdek, sarum darajasiga nisbatan izchil topilmalar mavjud 5-HIAA, serotonin metaboliti.[48] Monoaminlarning kamayib ketishiga olib keladigan farmakologik vositalar bilan o'tkazilgan tajribalar shuni ko'rsatdiki, bu tükenme sog'lom odamlarda ruhiy tushkunlikni keltirib chiqarmaydi.[49][50] Taqdim etilayotgan yana bir muammo shundaki, monoaminlarni yo'qotadigan dorilar aslida antidepressant xususiyatlariga ega bo'lishi mumkin. Bundan tashqari, ba'zilari depressiyani gipererotonerjik holat belgilashi mumkin, deb ta'kidlashdi.[51] Monoamin gipotezasi allaqachon cheklangan bo'lib, keng jamoatchilikka taqdim etilganda yanada soddalashtirilgan.[52]
Qabul qiluvchilarni bog'lash
2012 yildan boshlab nörotransmitter retseptorlari ekspressionida yoki MDD bilan og'rigan odamlarning miyasidagi funktsiyalarda farqlarni aniqlashga qaratilgan harakatlar. pozitron emissiya tomografiyasi (PET) bir-biriga mos kelmaydigan natijalarni ko'rsatdi. 2012 yilga kelib mavjud bo'lgan PET-ni ko'rish texnologiyasi va reaktivlaridan foydalangan holda, D1 retseptorlari MDD bilan kasallangan odamlarning striatumida kam ifoda etilgan bo'lishi mumkin. 5-HT1A retseptorlarning majburiy adabiyoti mos kelmaydi; ammo, meziotemporal korteksning umumiy pasayishiga to'g'ri keladi. 5-HT2A retseptorlari bilan bog'lanish MDD bilan og'rigan odamlarda tartibga solinmaganga o'xshaydi. 5-HTT bilan bog'lanish bo'yicha tadqiqotlar natijalari o'zgaruvchan, ammo MDD bilan kasallangan odamlarda yuqori darajalarni ko'rsatmoqda. Bilan natijalar D2 / D3 retseptorlari majburiy tadqiqotlar har qanday xulosa chiqarish uchun juda mos kelmaydi. Dalillar MDD bilan kasallangan odamlarda MAO faolligining oshishini qo'llab-quvvatlaydi va bu hatto belgi belgisi bo'lishi mumkin (davolanishga javoban o'zgartirilmaydi). Muskarinik retseptorlari bilan bog'lanish depressiyada kuchayganga o'xshaydi va ligand bilan bog'lanish dinamikasini hisobga olgan holda, xolinergik faollikni oshirishni taklif qiladi.[53]
Depressiyada retseptorlarni bog'lash bo'yicha to'rtta meta-tahlil o'tkazildi, ikkitasi serotonin tashuvchisi (5-HTT), bittasi 5-HTda1A, va boshqasi dopamin tashuvchisi (DAT). 5-HTT-dagi bir meta-tahlilda, ulanishning kamayganligi haqida xabar berilgan o'rta miya va amigdala, birinchisi kattaroq yoshga, ikkinchisi esa depressiya og'irligiga bog'liq.[54] V-jonli va in vivo jonli retseptorlari bilan bog'langan tadqiqotlar, shu jumladan 5-HTT bo'yicha yana bir meta-tahlil, in vivo jonli tadqiqotlar striatum, amigdala va o'rta miyada 5-HTT ning kamayganligini aniqlagan bo'lsa, o'limdan keyin o'tkazilgan tadqiqotlar hech qanday muhim birlashma topmadi.[55] 5-HT1A oldingi singulat korteksida, meziotemporal lobda, insula va hipokampusda kamayganligi aniqlandi, ammo amigdala yoki oksipital lobda emas. Eng ko'p ishlatiladigan 5-HT1A ligandlar endogen serotonin bilan almashtirilmaydi, bu retseptorlarning zichligi yoki yaqinligi kamayganligini ko'rsatadi.[56] Dopamin tashuvchisi bilan bog'lanish depressiyada o'zgarmaydi.[57]
Hissiy ishlov berish va asabiy zanjirlar
Hissiy tarafkashlik
MDD bilan kasallangan odamlar bir qator noto'g'ri fikrlarni namoyish etadilar hissiy ishlov berish Masalan, baxtli yuzlarni salbiy baholash tendentsiyasi va g'amgin iboralarga ko'proq e'tibor manbalarini ajratish tendentsiyasi.[58] Tushkunlikka tushgan odamlarda quvonchli, g'azablangan, jirkanch, qo'rqinchli va ajablanarli, ammo g'amgin bo'lmagan yuzlar tan olinishi yomonlashadi.[59] Funktsional neyroimaging turli xil miya mintaqalarining salbiy hissiy ogohlantirishlarga javoban giperaktivligini va ijobiy stimullarga javoban gipoaktivligini namoyish etdi. Bir meta-tahlil natijalariga ko'ra, tushkunlikka tushgan sub'ektlar chapdagi faollikni pasayganligini ko'rsatdilar dorsolateral prefrontal korteks va salbiy stimullarga javoban amigdalada faollik oshdi.[60] Boshqa bir meta-tahlilda keksa yoshdagi emas va nojo'ya kasalliklarga chalingan, sodda dori-darmon bo'lgan depressiv sub'ektlarning kichik guruhida hipokampus va talamus faolligining ko'tarilishi haqida xabar berilgan.[61] Antidepressantlarning terapevtik kechikishi antidepressantlarning kayfiyat o'zgarishiga olib keladigan hissiy qayta ishlashni o'zgartirishi natijasi deb taxmin qilingan. Buni o'tkir va subkronik kuzatuvlar qo'llab-quvvatlaydi SSRI ma'muriyat ijobiy yuzlarga javobni oshiradi.[62] Antidepressant bilan davolanish ruhiy holatga mos keluvchi tarafkashlikni teskari ta'sir qiladi limbik, prefrontal va fusiform joylar. dlPFC reaktsiyasi kuchayadi va amigdala reaktsiyasi salbiy his-tuyg'ularni qayta ishlash jarayonida susayadi, avvalgi yoki yuqoridan pastga regulyatsiya kuchaygan deb o'ylashadi. The fusiform girus va boshqalar vizual ishlov berish joylari ijobiy qayta ishlash tarafkashligini aks ettiradi deb hisoblanadigan antidepressant bilan ijobiy stimullarga kuchliroq javob bering.[63] Ushbu ta'sirlar serotonerjik yoki noradrenerjik antidepressantlarga xos emas, balki boshqa davolash usullarida ham uchraydi. chuqur miya stimulyatsiyasi.[64]
Nerv davrlari
Depressiyada funktsional neyroimagingning meta-tahlilidan birida emotsional ishlov berish tarafkashligini aks ettiradigan g'ayritabiiy asabiy faoliyat namunasi kuzatildi. Nazoratga nisbatan MDD bilan kasallangan odamlar giperaktivlikni ko'rsatdilar davrlar ichida muhim tarmoq (SN), dan tashkil topgan pulvinar yadrolari, insula va dorsal oldingi singulat korteksi (dACC), shuningdek, striatum va dlPFC dan tashkil topgan regulyatsion davrlarda faollikning pasayishi.[65]

Depressiyada erta biologik topilmalarni tushuntirish uchun limbik-kortikal model deb nomlangan neyroanatomik model taklif qilingan. Model depressiyaning o'ziga xos alomatlarini nevrologik anormallik bilan bog'lashga harakat qiladi. Ruminatsiya asosida yotgan amigdala faolligining ko'tarilishi taklif qilingan, chunki amigdalani stimulyatsiya qilish salbiy xotiralarni intruziv ravishda eslash bilan bog'liq. ACC ga bo'lingan pregenual (pgACC) va subgenual mintaqalar (sgACC), birinchisi elektrofizyologik jihatdan qo'rquv bilan bog'liq bo'lib, ikkinchisi metabolik tarzda sog'lom sub'ektlarda xafagarchilik bilan bog'liq. Yanal orbitofrontal va izolyatsion mintaqalarning giperaktivligi, lateral prefrontal mintaqalardagi anormalliklar bilan birga, moslashuvchan bo'lmagan hissiy reaktsiyalar asosida, mintaqalarni mukofotlashda rolini hisobga olgan holda taklif qilingan.[67][68] Ushbu model va boshqasi anormalliklarga ko'proq e'tibor qaratadigan "kortikal striatal model" deb nomlangan kortiko-bazal ganglion-talamo-kortikal halqa, so'nggi adabiyotlar tomonidan qo'llab-quvvatlandi. Kamaytirilgan striatal faollik, ko'tarilgan OFC faolligi va yuqori sgACC faolligi taklif qilingan modellarga mos keladigan topilmalar edi. Shu bilan birga, amigdala faolligi limbik-kortikal modelga zid ravishda kamayganligi haqida xabar berilgan. Bundan tashqari, faqat lateral prefrontal mintaqalar davolanish yo'li bilan modulyatsiya qilindi, bu prefrontal joylarning davlat belgilari (ya'ni, kayfiyatga bog'liq), subkortikal anormalliklar esa belgi belgilaridir (ya'ni, sezgirlikni aks ettiradi).[69]
Sovrin
Depressiya zo'ravonligi umuman olganda mukofot uchun xiralashgan asabiy javob bilan bog'liq bo'lmasa ham, anhedoniya da kamaytirilgan faollik bilan bevosita bog'liqdir mukofotlash tizimi.[70] Depressiyada mukofotni o'rganish mukofot va anhedoniyaning ta'rifi va kontseptsiyalaridagi heterojenlik bilan cheklangan. Anhedoniya keng ma'noda his qilish qobiliyatining pasayishi deb ta'riflanadi zavq, ammo anketalar va klinik baholash motivatsion "istak" va iste'mol qiluvchi "yoqtirish" ni kamdan-kam ajratib turadi. Bir qator tadqiqotlar shuni ko'rsatadiki, tushkunlikka tushgan mavzular ijobiy stimullarni kamroq ijobiy va kamroq uyg'otadi. Bundan tashqari, kabi tabiiy mukofotlarga javob saxaroza susaytirilmagan ko'rinadi. Umumiy ta'sirchan xiralashish depressiyadagi "anhedonik" alomatlarni tushuntirishi mumkin, chunki ijobiy va salbiy stimulyatorlarning meta-tahlilida intensivlikning pasaygan darajasi aniqlanadi.[71][72] Anhedoniya depressiyaning eng muhim alomati bo'lganligi sababli, tushkunlikka tushganlarni sog'lom sub'ektlar bilan to'g'ridan-to'g'ri taqqoslash, faollashuvining kuchayganligini ko'rsatadi subgenual oldingi singulat korteksi (sgACC), va faollashtirilishining kamayishi ventral striatum va xususan akumbens yadrosi (NAcc) ijobiy stimullarga javoban.[73] Garchi mukofot paradigmalarida kamaygan NAcc faolligini topish juda izchil bo'lsa ham, NAcc funktsional jihatdan turli xil neyronlardan tashkil topgan va kamaytirilgan qon-kislorod darajasiga bog'liq (BOLD) ushbu mintaqadagi signal turli xil narsalarni ko'rsatishi mumkin, shu jumladan afferent faollikni pasayishi yoki inhibitiv chiqindilarni kamaytirish.[74] Shunga qaramay, ushbu mintaqalar mukofotni qayta ishlashda muhim ahamiyatga ega va ularning depressiyadagi disfunktsiyasi yotadi anhedoniya. Serotonerjik antidepressantlar tomonidan aniq mo'ljallanmagan qoldiq anhedoniya, faollashishi bilan dopamin ajralishini oldini olish natijasida kelib chiqadi. 5-HT2C retseptorlari striatumda.[73] Medialda mukofotga javob orbitofrontal korteks (OFC) depressiyada susayadi, lateral OFC reaktsiyasi esa jazoga qadar kuchayadi. Yanal OFC mukofot yoki jazoning yo'qligiga barqaror munosabatni ko'rsatadi va o'zgaruvchan kutilmagan holatlarga javoban xatti-harakatlarni o'zgartirish uchun zarur deb hisoblanadi. LOFCda yuqori sezuvchanlik hayvonlarda o'rganilgan ojizlikka o'xshash ta'sir ko'rsatib, depressiyaga olib kelishi mumkin.[75]
SgACC-da ko'tarilgan javob - bu neyroimaging tadqiqotlarida bir qator paradigmalar yordamida mukofotga oid vazifalarni o'z ichiga olgan izchil topilma.[73][76][77] Davolash, shuningdek, sgACCdagi susaytirilgan faollik bilan bog'liq,[78] va sgACC ning kemiruvchilar gomologidagi neyronlarning inhibatsiyasi, infralimbik korteks (IL), antidepressant ta'sirini keltirib chiqaradi.[79] SgACC ning giperaktivligi mukofot yoki ijobiy stimulga somatik javobni susaytirishi orqali depressiyaga olib keladi deb taxmin qilingan.[80] Ning tadqiqotlaridan farqli o'laroq funktsional magnit-rezonans tomografiya vazifalar paytida sgACCda reaksiya, sgACCda dam olish metabolizmi kamayadi. Biroq, bu faqat depressiya bilan bog'liq bo'lgan sgACC hajmining sezilarli darajada pasayishini tuzatishda aniq bo'ladi; tizimli anormalliklarni hujayra darajasida yaqqol ko'rish mumkin, chunki neyropatologik tadqiqotlar sgACC hujayra belgilarining kamayganligi haqida xabar beradi. Drevets va boshqalarning ushbu topilmalaridan taklif qilingan depressiya modeli. sgACC faolligining pasayishi simpatik asab tizimining faolligini va HPA o'qining teskari aloqasini keltirib chiqaradi.[81] SgACCdagi faollik depressiyada ham sabab bo'lmasligi mumkin, chunki emotsional regulyatsiya paytida tushkunlikka tushgan sub'ektlarda neyro tasvirlashni o'rgangan bitta sharh mualliflari yuqori sgACC faolligining namunasi depressiyada avtomatik hissiy reaktsiyalarni modulyatsiya qilish zarurligini aks ettirgan deb taxmin qilishdi. Ijobiy emotsional ishlov berish jarayonida kengroq sgACC va umumiy prefrontal yollash ijobiy his-tuyg'ularga subkortikal javob va anhedoniya bilan bog'liq edi. Bu mualliflar tomonidan prefrontal korteksni haddan tashqari jalb qilish orqali ijobiy his-tuyg'ularni kamaytirishni aks ettirish uchun talqin qilingan.[82]
Neyroanatomiya
Katta depressiya buzilishi bo'lgan odamlarda bir qator neyroimaging topilmalari haqida doimiy ravishda xabar berilgan bo'lsa-da, depressiv populyatsiyalarning bir xilligi bu topilmalarni izohlashda qiyinchiliklarni keltirib chiqaradi. Masalan, populyatsiyalar bo'yicha o'rtacha ko'rsatkich kichik guruhga tegishli topilmalarni yashirishi mumkin; depressiyada dlPFC faolligining pasayishi qayd etilsa, kichik guruhda dlPFC faolligi oshishi mumkin. O'rtacha, shuningdek sub'ektlarning kichik guruhida mavjud bo'lgan hipokampal hajmlarning kamayishi kabi statistik jihatdan muhim natijalarni berishi mumkin.[83] Ushbu muammolar va boshqalar, shu jumladan depressiyaning uzunlamasına tutarlılığı tufayli, ko'pgina asab modellari, ehtimol, barcha depressiyalarga tatbiq etilmaydi.[69]
Strukturaviy neyroimaging

Yordamida amalga oshirilgan meta-tahlillar urug'larga asoslangan d xaritalash bir qator frontal mintaqalarda kulrang moddalar kamayganligi haqida xabar berishdi. Erta boshlangan umumiy depressiyani meta-tahlilida ikki tomonlama kulrang moddalar kamayganligi haqida xabar berilgan oldingi singulat korteksi (ACC) va dorsomedial prefrontal korteks (dmPFC).[85] Birinchi epizod depressiyasida o'tkazilgan meta-tahlil natijasida dori-darmonlarsiz va birlashgan populyatsiyalarda kulrang moddalarning kamayishining aniq namunalari kuzatildi; dorilarsiz depressiya o'ngdagi pasayish bilan bog'liq edi dorsolateral prefrontal korteks, to'g'ri amigdala va to'g'ri pastki temporal girus; Dori-darmonlarni bepul va davolovchi ruhiy tushkunlik kombinatsiyasini tahlil qilish natijasida chap insula, o'ng qo'shimcha vosita sohasi va o'ng o'rta temporal girusda pasayish aniqlandi.[86] Tibbiy va dori-darmonlardan tashqari populyatsiyalarni ajratib ko'rsatadigan yana bir sharh, MDDning birinchi epizodi bo'lgan odamlar bilan cheklanmagan bo'lsa ham, ikki tomonlama yuqori, o'ng o'rta va chap pastki frontal girusda birlashtirilgan populyatsiyada kamayish aniqlandi. parahippokampus. Talamik va ACC kulrang moddalarining ko'payishi navbati bilan dorilarsiz va dori-darmonli populyatsiyalarda qayd etilgan.[87] "Aktivizatsiya ehtimolini baholash" yordamida o'tkazilgan meta-tahlil parakingulyat korteks, dACC va amigdalada pasayishlar haqida xabar berdi.[88]
Statistik parametrik xaritalash yordamida bitta meta-tahlil ACC, medial prefrontal korteks, pastki frontal girus, gipokampus va talamusda kamaytirilgan kulrang moddalarning oldingi natijalarini takrorladi; ammo OFKdagi pasayishlar va ventromedial prefrontal korteks kulrang moddalar haqida ham xabar berilgan.[89]
ENIGMA konsortsiumidan depressiya bo'yicha ikkita tadqiqot nashr etildi, biri kortikal qalinligi, ikkinchisi subkortikal hajmi. Kortikal qalinlikning kamayishi ikki tomonlama OFC, ACC, insula, o'rta temporal giru, fusiform giri va orqa singulat kortekslarida qayd etilgan, yuza tanqisligi medial oksipital, pastki parietal, orbitofrontal va prekentral mintaqalarda topilgan.[90] Subkortikal anormallik, shu jumladan hipokampus va amigdala hajmining pasayishi, bu ayniqsa erta boshlangan depressiyada aniqlandi.[91]
Tadqiqotlarni baholashda bir nechta meta-tahlillar o'tkazildi oq materiya yaxlitlikdan foydalanish fraksiyonel anizotropiya (FA). Qisqartirilgan FA haqida xabar berilgan korpus kallosum (CC) ikkala birinchi epizodda ham dorilar sodda,[93][94] va umumiy depressiv populyatsiyalar.[92][95] CCni kamaytirish darajasi har bir o'quvdan farq qiladi. Ilgari antidepressantlarni qabul qilmagan MDD bilan kasallangan odamlarda faqat CC tanasida pasayish kuzatilganligi haqida xabar berilgan[93] va faqat CC genu-da.[94] Boshqa tomondan, umumiy MDD namunalarida CC tanasida pasayish kuzatilgan,[94] CC tanasi va genu,[92] va faqat CC genu.[95] FA-ning kamayishi haqida ham xabar berilgan ichki kapsulaning oldingi qismi (ALIC)[93][92] va yuqori bo'ylama fasciculus.[93][94]
Funktsional neyroimaging
Dam olish holati holatini o'rganish bir qator dam olish holati ko'rsatkichlarini, shu jumladan mintaqaviy bir xillik (ReHO), past chastotali dalgalanmalar amplitudasi (ALFF), past chastotali dalgalanmalarning fraksiyonel amplituda (fALFF) dan foydalangan. arterial spin yorlig'i (ASL) va pozitron emissiya tomografiyasi mintaqaviy miya qon oqimi yoki metabolizmining choralari.
ALFF va FALFF dan foydalangan holda olib borilgan tadqiqotlar ACC faolligining ko'tarilishini qayd etdi, birinchisi asosan ventral topilmalar haqida, ikkinchisi esa dorsal topilmalar haqida xabar berdi.[96] ALFF va CBF tadqiqotlarini birgalikda tahlillari chap insulada birlashdi, ilgari davolanmagan odamlar insula faolligini oshirdilar. Baland kaudat Shuningdek, CBF haqida xabar berilgan[97] Dam olish faoliyatining ko'plab ko'rsatkichlarini birlashtirgan meta-tahlilda oldingi singulat, striatal va talamik faollik ko'tarilganligi va chap insula, post-markaziy girus va fusiform girus faoliyati kamayganligi haqida xabar berilgan.[98] PET / SPECT dam olish holatlarini o'rganish bo'yicha aktivizatsiya ehtimolini (ALE) meta-tahlilida chap insula, oldingi va dorsal oldingi singulat korteksidagi faollik kamayganligi va talamus, kaudat, oldingi hipokampus va amigdaladagi faollik ko'tarilganligi haqida xabar berilgan.[99] PET / SPECT tadqiqotlarining ALE meta-tahlili bilan taqqoslaganda, ko'p yadroli zichlik tahlilidan foydalangan holda, giperaktivlik faqat pulvinar yadrolari talamus.[65]
Miya mintaqalari
MDD bilan kasallangan odamlarning miyasida olib borilgan tadqiqotlar, odatda, miyaning ko'p qismlari o'rtasida buzilgan o'zaro ta'sir modellarini ko'rsatadi. Miyaning bir nechta sohalari depressiya biologiyasini to'liqroq o'rganishga qaratilgan tadqiqotlarda ishtirok etadi:
Subgenual singulat
Tadqiqotlar shuni ko'rsatdiki Brodmann maydoni 25 subgenual singulat deb ham ataladigan metabolik jihatdan haddan tashqari faol davolashga chidamli depressiya. Ushbu mintaqa juda boy serotonin tashuvchilar va shunga o'xshash hududlarni o'z ichiga olgan ulkan tarmoq uchun hokim sifatida qaraladi gipotalamus va miya sopi, bu tuyadi va uyquning o'zgarishiga ta'sir qiladi; The amigdala va insula, kayfiyat va tashvishga ta'sir qiladigan; The gipokampus, bu xotirani shakllantirishda muhim rol o'ynaydi; va ba'zi qismlari Frontal korteks o'z-o'zini hurmat qilish uchun javobgardir. Shunday qilib, ushbu hududdagi bezovtaliklar yoki ushbu hududning odatdagidan kichikroq bo'lishi depressiyani keltirib chiqaradi. Miyaning chuqur stimulyatsiyasi davolashga chidamli depressiyaga chalingan odamlarda uning faoliyatini kamaytirish uchun ushbu mintaqaga yo'naltirilgan.[100]:576–578[101]
Prefrontal korteks
Bir tekshiruvda hipoaktivlik haqida xabar berilgan prefrontal korteks depressiyaga chalinganlarning nazorati bilan taqqoslaganda.[102] Prefrontal korteks hissiy qayta ishlash va tartibga solishda ishtirok etadi va bu jarayonning disfunktsiyasi depressiya etiologiyasida ishtirok etishi mumkin. Antidepressantlarni davolash bo'yicha bir tadqiqot antidepressantlarni qabul qilishga javoban PFC faolligini oshirganligini aniqladi.[103] 2012 yilda nashr etilgan meta-tahlillardan birida prefrontal korteks sohalari MDD bilan kasallangan odamlarning salbiy ta'siriga javoban gipoaktiv bo'lganligi aniqlandi.[65] Bir tadqiqot shuni ko'rsatdiki, prefrontal korteks sohalari MDD bilan kasallangan odamlarda gipoaktiv bo'lib ko'rinadigan dorsal va tug'ma singulat, ikki tomonlama o'rta frontal girus, insula va yuqori vaqtinchalik girusni o'z ichiga olgan mintaqalar tarmog'ining bir qismidir. Biroq, mualliflar istisno mezonlari, izchillik yo'qligi va kichik namunalar natijalarni cheklashi haqida ogohlantirdi.[99]
Amigdala
Amigdala, hissiyotlarni qayta ishlash bilan bog'liq tuzilish, katta depressiya buzilishi bo'lganlarda giperaktiv bo'lib ko'rinadi.[101] Dori-darmon bilan davolanmagan depressiyadagi amigdala mayda bo'lgan, ammo umumiy ma'lumotlar depressiya va sog'lom odamlar o'rtasida farq yo'q.[104] Emotsional ishlov berish paytida o'ng amigdala chapga qaraganda faolroq bo'ladi, ammo bilish vazifalari davomida farqlar bo'lmaydi, tinch holatda faqat chap amigdala ko'proq giperaktiv ko'rinadi.[105] Biroq, bitta tadqiqotda, emotsional ishlov berish vazifalari paytida amigdala faoliyatida farq yo'qligi aniqlandi.[106]
Gipokampus
Depressiya paytida hipokampning atrofiyasi kuzatilgan, bu hayvonlardagi stress va neyrogenez modellariga mos keladi.[107][108]
Stress depressiya va depressiyaga o'xshash simptomlarni miyaning bir nechta asosiy mintaqalarida monoaminerjik o'zgarishlar hamda hipokampal neyrogenezda bostirish orqali keltirib chiqarishi mumkin.[109] Bu hissiyot va idrok bilan bog'liq miya mintaqalarida va HPA o'qi disfunktsiyasida o'zgarishlarga olib keladi. Disfunktsiya orqali stressning ta'siri kuchayishi mumkin, shu jumladan uning 5-HTga ta'siri. Furthermore, some of these effects are reversed by antidepressant action, which may act by increasing hippocampal neurogenesis. This leads to a restoration in HPA activity and stress reactivity, thus restoring the deleterious effects induced by stress on 5-HT.[110]
The gipotalamus-gipofiz-buyrak usti o'qi ning zanjiri endokrin structures that are activated during the body's response to stressors of various sorts. The HPA axis involves three structure, the hypothalamus which release CRH that stimulates the pituitary gland to release ACTH which stimulates the adrenal glands to release cortisol. Cortisol has a negative feedback effect on the pituitary gland and hypothalamus. In people with MDD the often shows increased activation in depressed people, but the mechanism behind this is not yet known.[111] Increased basal cortisol levels and abnormal response to dexamethasone challenges have been observed in people with MDD.[112] Early life stress has been hypothesized as a potential cause of HPA dysfunction.[113][114] HPA axis regulation may be examined through a dexamethasone suppression tests, which tests the feedback mechanisms. Non-suppression of dexamethasone is a common finding in depression, but is not consistent enough to be used as a diagnostic tool.[115] HPA axis changes by be responsible for some of the changes such as decreased bone mineral density and increased weight found in people with MDD. One drug, ketoconazole, currently under development has shown promise in treating MDD.[116]
Hippocampal Neurogenesis
Kamaytirilgan gipokampal neurogenesis leads to a reduction in hippocampal volume. A genetically smaller hippocampus has been linked to a reduced ability to process psixologik travma and external stress, and subsequent predisposition to psychological illness.[117] Depression without familial risk or childhood trauma has been linked to a normal hippocampal volume but localised dysfunction.[118]
Animal Models
A number of animal models exist for depression, but they are limited in that depression involves primarily subjective emotional changes. However, some of these changes are reflected in physiology and behavior, the latter of which is the target of many animal models. These models are generally assessed according to four facets of validity; the reflection of the core symptoms in the model; the predictive validity of the model; the validity of the model with regard to human characteristics of etiology;[119] and the biological plausibility.[120][121]
Different models for inducing depressive behaviors have been utilized; neuroanatomical manipulations such as olfactory bulbectomy or circuit specific manipulations with optogenetics; genetic models such as 5-HT1A knockout or selectively bred animals;[119] models involving environmental manipulation associated with depression in humans, including chronic mild stress, early life stress and learned helplessness.[122] The validity of these models in producing depressive behaviors may be assessed with a number of behavioral tests. Anhedonia and motivational deficits may, for example, be assessed via examining an animal's level of engagement with rewarding stimuli such as sucrose or intracranial self-stimulation. Anxious and irritable symptoms may be assessed with exploratory behavior in the presence of a stressful or novelty environment, such as the open field test, novelty suppressed feeding, or the elevated plus-maze. Fatigue, psychomotor poverty, and agitation may be assessed with locomotor activity, grooming activity, and open field tests.
Animal models possess a number of limitations due to the nature of depression. Some core symptoms of depression, such as rumination, low self-esteem, guilt, and depressed mood cannot be assessed in animals as they require subjective reporting.[121] From an evolutionary standpoint, the behavior correlates of defeats of loss are thought to be an adaptive response to prevent further loss. Therefore, attempts to model depression that seeks to induce defeat or despair may actually reflect adaption and not disease. Furthermore, while depression and anxiety are frequently comorbid, dissociation of the two in animal models is difficult to achieve.[119] Pharmacological assessment of validity is frequently disconnected from clinical pharmacotherapeutics in that most screening tests assess acute effects, while antidepressants normally take a few weeks to work in humans.[123]
Neurocircuits
Regions involved in reward are common targets of manipulation in animal models of depression, including the nucleus accumbens (NAc), ventral tegmental area (VTA), ventral pallidum (VP), lateral habenula (LHb) va medial prefrontal korteks (mPFC). Tentative fMRI studies in humans demonstrate elevated LHb activity in depression.[124] The lateral habenula projects to the RMTg to drive inhibition of dopamine neurons in the VTA during omission of reward. In animal models of depression, elevated activity has been reported in LHb neurons that project to the ventral tegmental maydon (ostensibly reducing dopamine release). The LHb also projects to aversion reactive mPFC neurons, which may provide an indirect mechanism for producing depressive behaviors.[125] Learned helplessness induced potentiation of LHb synapses are reversed by antidepressant treatment, providing predictive validity.[124] A number of inputs to the LHb have been implicated in producing depressive behaviors. Silencing GABAergic projections from the NAc to the LHb reduces conditioned place preference induced in social aggression, and activation of these terminals induces CPP. Ventral pallidum firing is also elevated by stress induced depression, an effect that is pharmacologically valid, and silencing of these neurons alleviates behavioral correlates of depression.[124] Tentative in vivo evidence from people with MDD suggests abnormalities in dopamine signalling.[126] This led to early studies investigating VTA activity and manipulations in animal models of depression. Massive destruction of VTA neurons enhances depressive behaviors, while VTA neurons reduce firing in response to chronic stress. However, more recent specific manipulations of the VTA produce varying results, with the specific animal model, duration of VTA manipulation, method of VTA manipulation, and subregion of VTA manipulation all potentially leading to differential outcomes.[127] Stress and social defeat induced depressive symptoms, including anhedonia, are associated with potentiation of excitatory inputs to Dopamine D2 receptor-expressing medium spiny neurons (D2-MSNs) and depression of excitatory inputs to Dopamine D1 receptor-expressing medium spiny neurons (D1-MSNs). Optogenetic excitation of D1-MSNs alleviates depressive symptoms and is rewarding, while the same with D2-MSNs enhances depressive symptoms. Excitation of glutaminergic inputs from the ventral hippocampus reduces social interactions, and enhancing these projections produces susceptibility to stress-induced depression.[127] Manipulations of different regions of the mPFC can produce and attenuate depressive behaviors. For example, inhibiting mPFC neurons specifically in the intralimbic cortex attenuates depressive behaviors. The conflicting findings associated with mPFC stimulation, when compared to the relatively specific findings in the infralimbic cortex, suggest that the prelimbic cortex and infralimbic cortex may mediate opposing effects.[79] mPFC projections to the raphe nuclei are largely GABAergic and inhibit the firing of serotonergic neurons. Specific activation of these regions reduce immobility in the forced swim test but do not affect open field or forced swim behavior. Inhibition of the raphe shifts the behavioral phenotype of uncontrolled stress to a phenotype closer to that of controlled stress.[128]
Altered neuroplasticity
Recent studies have called attention to the role of altered neyroplastiklik in depression. A review found a convergence of three phenomena:
- Chronic stress reduces synaptic and dendritic plasticity
- Depressed subjects show evidence of impaired neuroplasticity (e.g. shortening and reduced complexity of dendritic trees)
- Anti-depressant medications may enhance neuroplasticity at both a molecular and dendritic level.
The conclusion is that disrupted neuroplasticity is an underlying feature of depression, and is reversed by antidepressants.[129]
Blood levels of BDNF in people with MDD increase significantly with antidepressant treatment and correlate with decrease in symptoms.[130] Post mortem studies and rat models demonstrate decreased neuronal density in the prefrontal cortex thickness in people with MDD. Rat models demonstrate histological changes consistent with MRI findings in humans, however studies on neurogenesis in humans are limited. Antidepressants appear to reverse the changes in neurogenesis in both animal models and humans.[131]
Inflammation and oxidative stress
Various review have found that general inflammation may play a role in depression.[132][133] One meta analysis of cytokines in people with MDD found increased levels of pro-inflammatory IL-6 and TNF-a levels relative to controls.[134] The first theories came about when it was noticed that interferon therapy caused depression in a large number of people receiving it.[135] Meta analysis on cytokine levels in people with MDD have demonstrated increased levels of Il-1, Il-6, C-reaktiv oqsil, lekin emas Il-10.[136][137] Increased numbers of T-Cells presenting activation markers, levels of neopterin, IFN gamma, sTNFR, and IL-2 receptors have been observed in depression.[138] Various sources of inflammation in depressive illness have been hypothesized and include trauma, sleep problems, diet, smoking and obesity.[139] Cytokines, by manipulating neurotransmitters, are involved in the generation of sickness behavior, which shares some overlap with the symptoms of depression. Neurotransmitters hypothesized to be affected include dopamine and serotonin, which are common targets for antidepressant drugs. Induction of indolamine-2,3 dioxygenease by cytokines has been proposed as a mechanism by which immune dysfunction causes depression.[140] One review found normalization of cytokine levels after successful treatment of depression.[141] A meta analysis published in 2014 found the use of anti-inflammatory drugs such as NSAIDs and investigational cytokine inhibitors reduced depressive symptoms.[142] Exercise can act as a stressor, decreasing the levels of IL-6 and TNF-a and increasing those of IL-10, an anti-inflammatory cytokine.[143]
Inflammation is also intimately linked with metabolic processes in humans. For example, low levels of Vitamin D have been associated with greater risk for depression.[144] The role of metabolic biomarkers in depression is an active research area. Recent work has explored the potential relationship between plasma sterols and depressive symptom severity.[145]
A marker of DNA oxidation, 8-okso-2'-deoksiguanozin, has been found to be increased in both the plasma and urine of people with MDD. This along with the finding of increased F2-isoprostanes levels found in blood, urine and cerebrospinal fluid indicate increased damage to lipids and DNA in people with MDD. Studies with 8-Oxo-2' Deoxyguanosine varied by methods of measurement and type of depression, but F2-Isoprostane level was consistent across depression types. Authors suggested lifestyle factors, dysregulation of the HPA axis, immune system and autonomics nervous system as possible causes.[146] Another meta-analysis found similar results with regards to oxidative damage products as well as decreased oxidative capacity.[147] Oxidative DNA damage may play a role in MDD.[148]
Mitochondrial Dysfunction:
Increased markers of oxidative stress relative to controls have been found in people with MDD.[149] These markers include high levels of RNS va ROS which have been shown to influence chronic inflammation, damaging the elektron transport zanjiri and biochemical cascades in mitoxondriya. This lowers the activity of enzymes in the respiratory chain resulting in mitochondrial dysfunction.[150] The brain is a highly energy-consuming and has little capacity to store glucose as glycogen and so depends greatly on mitochondria. Mitochondrial dysfunction has been linked to the dampened neyroplastiklik observed in depressed brains.[151]
Large-scale brain network theory
Instead of studying one brain region, studying large scale brain networks is another approach to understanding psychiatric and neurological disorders,[152] supported by recent research that has shown that multiple brain regions are involved in these disorders. Understanding the disruptions in these networks may provide important insights into interventions for treating these disorders. Recent work suggests that at least three large-scale brain networks are important in psychopathology:[152]
Central executive network
The central executive network is made up of fronto-parietal regions, including dorsolateral prefrontal cortex and lateral posterior parietal cortex.[153][154] This network is involved in high level kognitiv funktsiyalar such as maintaining and using information in ishlaydigan xotira, problem solving, and decision making.[152][155] Deficiencies in this network are common in most major psychiatric and neurological disorders, including depression.[156][157] Because this network is crucial for everyday life activities, those who are depressed can show impairment in basic activities like test taking and being decisive.[158]
Standart rejimdagi tarmoq
The standart rejimdagi tarmoq includes hubs in the prefrontal cortex and posterior cingulate, with other prominent regions of the network in the medial temporal lobe and angular gyrus.[152] The default mode network is usually active during mind-wandering and thinking about social situations. In contrast, during specific tasks probed in cognitive science (for example, simple attention tasks), the default network is often deactivated.[159][160] Research has shown that regions in the default mode network (including medial prefrontal cortex and posterior cingulate) show greater activity when depressed participants ruminate (that is, when they engage in repetitive self-focused thinking) than when typical, healthy participants ruminate.[161] People with MDD also show increased connectivity between the default mode network and the subgenual cingulate and the adjoining ventromedial prefrontal cortex in comparison to healthy individuals, individuals with dementia or with autism. Numerous studies suggest that the subgenual cingulate plays an important role in the dysfunction that characterizes major depression.[162] The increased activation in the default mode network during rumination and the atypical connectivity between core default mode regions and the subgenual cingulate may underlie the tendency for depressed individual to get "stuck" in the negative, self-focused thoughts that often characterize depression.[163] However, further research is needed to gain a precise understanding of how these network interactions map to specific symptoms of depression.
Salience tarmog'i
The salience network is a cingulate-frontal operculum network that includes core nodes in the anterior cingulate and anterior insula.[153] A keskinlik network is a large-scale brain network involved in detecting and orienting the most pertinent of the external stimuli and internal events being presented.[152] Individuals who have a tendency to experience negative emotional states (scoring high on measures of nevrotikizm ) show an increase in the right anterior insula during decision-making, even if the decision has already been made.[164] This atypically high activity in the right anterior insula is thought to contribute to the experience of negative and worrisome feelings.[165] In major depressive disorder, anxiety is often a part of the emotional state that characterizes depression.[166]
Shuningdek qarang
Adabiyotlar
- ^ Anglin, Rebecca E.; Tarnopolskiy, Mark A.; Mazurek, Michael F.; Rosebush, Patricia I. (January 2012). "The Psychiatric Presentation of Mitochondrial Disorders in Adults". Nöropsikiyatriya va klinik nevrologiya jurnali. 24 (4): 394–409. doi:10.1176/appi.neuropsych.11110345. ISSN 0895-0172. PMID 23224446.
- ^ CARROLL, BERNARD J. (October 2004). "Psychoneuroendocrinology: The Scientific Basis of Clinical Practice. Edited by O. M. Wolkowitz and A. J. Rothschild. (Pp. 606; $73.95; ISBN 0-88048-857-3 pb.) American Psychiatric Publishing, Inc.: Arlington, Virginia, 2003". Psixologik tibbiyot. 34 (7): 1359–1360. doi:10.1017/S0033291704213678. ISSN 0033-2917.
- ^ Kupfer DJ, Frank E, Phillips ML (17 March 2012). "Major depressive disorder: new clinical, neurobiological, and treatment perspectives". Lanset. 379 (9820): 1045–55. doi:10.1016/S0140-6736(11)60602-8. PMC 3397431. PMID 22189047.
- ^ aan het Rot M, Mathew SJ, Charney DS (3 February 2009). "Neurobiological mechanisms in major depressive disorder". Kanada tibbiyot birlashmasi jurnali. 180 (3): 305–13. doi:10.1503/cmaj.080697. PMC 2630359. PMID 19188629.
- ^ Levinson, Douglas F.; Nichols, Walter E. (2018). "24. Genetics of Depression". In Charney, Dennis S.; Sklar, Pamela; Buxbaum, Joseph D.; Nestler, Eric J. (eds.). Charney & Nestlers Neurobiology of Mental Illness (5-nashr). Nyu-York: Oksford universiteti matbuoti. p. 310.
- ^ Nierenberg, AA (2009). "Serotoninni qabul qilish oqsilini kodlaydigan gen uchun promotor mintaqaning qisqa qo'li to'g'risida uzoq ertak". CNS spektrlari. 14 (9): 462–3. doi:10.1017 / s1092852900023506. PMID 19890228.
- ^ Risch, N; Herrell, R; Lehner, T; Liang, KY; Eaves, L; Hoh, J; Griem, A; Kovacs, M; Ott, J; Merikangas, KR (17 June 2009). "Serotonin tashuvchisi geni (5-HTTLPR), stressli hayotiy hodisalar va depressiya xavfi o'rtasidagi o'zaro ta'sir: meta-tahlil". JAMA. 301 (23): 2462–71. doi:10.1001 / jama.2009.878. PMC 2938776. PMID 19531786.
- ^ Munafò, MR; Durrant, C; Lewis, G; Flint, J (1 February 2009). "Serotonin tashuvchisi lokusidagi Gen X muhitining o'zaro ta'siri". Biologik psixiatriya. 65 (3): 211–9. doi:10.1016 / j.biopsych.2008.06.009. PMID 18691701.
- ^ Karg, K; Burmeister, M; Shedden, K; Sen, S (May 2011). "Serotonin tashuvchisi targ'ibotchining varianti (5-HTTLPR), stress va depressiya meta-tahlili qayta ko'rib chiqildi: genetik moderatsiya dalili". Umumiy psixiatriya arxivi. 68 (5): 444–54. doi:10.1001 / archgenpsychiatry.2010.189. PMC 3740203. PMID 21199959.
- ^ Taylor, AE; Munafò, MR (31 May 2016). "Triangulating meta-analyses: the example of the serotonin transporter gene, stressful life events and major depression". BMC Psychology. 4 (1): 23. doi:10.1186/s40359-016-0129-0. PMC 4886450. PMID 27240561.
- ^ Bleys, D; Lyuyten, P; Soenens, B; Claes, S (15 January 2018). "Gene-environment interactions between stress and 5-HTTLPR in depression: A meta-analytic update" (PDF). Affektiv buzilishlar jurnali. 226: 339–345. doi:10.1016/j.jad.2017.09.050. PMID 29031184.
- ^ Fanelli G, Serretti A (2019). "The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis". Prog Neuropsychopharmacol Biol Psixiatriyasi. 88: 375–387. doi:10.1016/j.pnpbp.2018.08.007. PMID 30125622.CS1 maint: mualliflar parametridan foydalanadi (havola)
- ^ Levinson, D. (2006). "The genetics of depression: a review". Biologik psixiatriya. 60 (2): 84–92. doi:10.1016/j.biopsych.2005.08.024. PMID 16300747.
- ^ Dwivedi Y (2009). "Brain-derived neurotrophic factor: role in depression and suicide". Nöropsikiyatrik davolash. 5: 433–49. doi:10.2147/NDT.S5700. PMC 2732010. PMID 19721723.
- ^ a b Krishnan, V .; Nestler, E. (2008). "The molecular neurobiology of depression". Tabiat. 455 (7215): 894–902. Bibcode:2008Natur.455..894K. doi:10.1038/nature07455. PMC 2721780. PMID 18923511.
- ^ Pezawas, L.; Meyer-Lindenberg, A.; Goldman, A. L.; Verchinski, B. A .; Chen, G.; Kolachana, B. S.; Egan, M. F.; Mattey, V. S.; Hariri, A. R.; Weinberger, D. R. (2008). "Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression". Molekulyar psixiatriya. 13 (7): 709–716. doi:10.1038/mp.2008.32. PMID 18347599.
- ^ Converge Consortium; Bigdeli, Tim B.; Kretzschmar, Warren; Li, Yihan; Liang, Jieqin; Song, Li; Hu, Jingchu; Li, Qibin; Jin, Vey; Hu, Zhenfei; Wang, Guangbiao; Wang, Linmao; Qian, Puyi; Liu, Yuan; Tszyan, Tao; Lu, Yao; Zhang, Xiuqing; Yin, sen; Li, Yingrui; Xu, Xun; Gao, Jingfang; Reimers, Mark; Webb, Todd; Rayli, Brien; Bacanu, Silviu; Peterson, Roseann E.; Chen, Yiping; Zhong, Hui; Liu, Zhengrong; va boshq. (2015). "Genomning siyrak ketma-ketligi yirik depressiya buzilishi uchun ikkita joyni aniqlaydi". Tabiat. 523 (7562): 588–91. Bibcode:2015Natur.523..588C. doi:10.1038 / tabiat 14659. PMC 4522619. PMID 26176920.
- ^ Smoller, Jordan W (2015). "The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders". Nöropsikofarmakologiya. 41 (1): 297–319. doi:10.1038/npp.2015.266. PMC 4677147. PMID 26321314.
- ^ Zhao, Xiaofeng; Huang, Yinglin; Ma, Hui; Jin, Qiu; Vang, Yuan; Zhu, Gang (15 August 2013). "Association between major depressive disorder and the norepinephrine transporter polymorphisms T-182C and G1287A: a meta-analysis". Affektiv buzilishlar jurnali. 150 (1): 23–28. doi:10.1016/j.jad.2013.03.016. ISSN 1573-2517. PMID 23648227.
- ^ Lohoff, Falk W. (6 December 2016). "Overview of the Genetics of Major Depressive Disorder". Hozirgi psixiatriya hisobotlari. 12 (6): 539–546. doi:10.1007/s11920-010-0150-6. ISSN 1523-3812. PMC 3077049. PMID 20848240.
- ^ López-León, S.; Janssens, A. C. J. W.; González-Zuloeta Ladd, A. M.; Del-Favero, J.; Claes, S. J.; Oostra, B. A.; van Duijn, C. M. (1 August 2008). "Meta-analyses of genetic studies on major depressive disorder". Molekulyar psixiatriya. 13 (8): 772–785. doi:10.1038/sj.mp.4002088. ISSN 1476-5578. PMID 17938638.
- ^ a b Karlson, Nil R. (2013). Xulq-atvor fiziologiyasi (11-nashr). Boston: Pearson. 578-582 betlar. ISBN 978-0-205-23939-9. OCLC 769818904.
- ^ a b v Adrien J.. Neurobiological bases for the relation between sleep and depression. Uyquga oid dorilarni ko'rib chiqish. 2003;6(5):341–51. doi:10.1053/smrv.2001.0200. PMID 12531125.
- ^ a b Terman M. Evolving applications of light therapy. Uyquga oid dorilarni ko'rib chiqish. 2007;11(6):497–507. doi:10.1016/j.smrv.2007.06.003. PMID 17964200.
- ^ Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Uyquga oid dorilarni ko'rib chiqish. 2007;11(6):509–22. doi:10.1016/j.smrv.2007.06.004. PMID 17689120.
- ^ Zhai, Long; Chjan, Xua; Zhang, Dongfeng (1 September 2015). "Sleep Duration and Depression Among Adults: A Meta-Analysis of Prospective Studies". Depressiya va tashvish. 32 (9): 664–670. doi:10.1002/da.22386. ISSN 1520-6394. PMID 26047492.
- ^ Germain, Anne; Kupfer, David J. (6 December 2016). "Circadian rhythm disturbances in depression". Inson psixofarmakologiyasi. 23 (7): 571–585. doi:10.1002/hup.964. ISSN 0885-6222. PMC 2612129. PMID 18680211.
- ^ Savitz, Jonathan B.; Drevets, Wayne C. (1 April 2013). "Neuroreceptor imaging in depression". Kasallikning neyrobiologiyasi. 52: 49–65. doi:10.1016/j.nbd.2012.06.001. ISSN 1095-953X. PMID 22691454.
- ^ Carlson, Neil R. (2005). Foundations of Physiological Psychology (6-nashr). Boston: Pearson A and B. p.108. ISBN 978-0-205-42723-9. OCLC 60880502.
- ^ Marchand; Valentina; Jensen. "Neurobiology of Mood disorders". Kasalxona shifokori: 17–26.
- ^ Hjorth, S; Bengtsson, HJ; Kullberg, A; Carlzon, D; Peilot, H; Auerbach, SB (June 2000). "Serotonin autoreceptor function and antidepressant drug action". Psixofarmakologiya jurnali (Oksford, Angliya). 14 (2): 177–85. doi:10.1177/026988110001400208. PMID 10890313.
- ^ a b COWEN, P (September 2008). "Serotonin and depression: pathophysiological mechanism or marketing myth?". Farmakologiya fanlari tendentsiyalari. 29 (9): 433–436. doi:10.1016/j.tips.2008.05.004. PMID 18585794.
- ^ a b Harmer, CJ (November 2008). "Serotonin and emotional processing: does it help explain antidepressant drug action?". Neyrofarmakologiya. 55 (6): 1023–8. doi:10.1016/j.neuropharm.2008.06.036. PMID 18634807.
- ^ Dunlop, Boadie W.; Nemeroff, Charles B. (1 April 2007). "The Role of Dopamine in the Pathophysiology of Depression". Umumiy psixiatriya arxivi. 64 (3): 327–37. doi:10.1001/archpsyc.64.3.327. ISSN 0003-990X. PMID 17339521.
- ^ Willner, Paul (1 December 1983). "Dopamine and depression: A review of recent evidence. I. Empirical studies". Miya tadqiqotlari bo'yicha sharhlar. 6 (3): 211–224. doi:10.1016/0165-0173(83)90005-X. PMID 6140979.
- ^ a b HASLER, GREGOR (4 December 2016). "Pathophysiology of Depression: Do We Have Any Solid Evidence of Interest to Clinicians?". Jahon psixiatriyasi. 9 (3): 155–161. doi:10.1002/j.2051-5545.2010.tb00298.x. ISSN 1723-8617. PMC 2950973. PMID 20975857.
- ^ Kunugi, Hiroshi; Hori, Hiroaki; Ogawa, Shintaro (1 October 2015). "Biochemical markers subtyping major depressive disorder". Psixiatriya va klinik nevrologiya. 69 (10): 597–608. doi:10.1111/pcn.12299. ISSN 1440-1819. PMID 25825158.
- ^ Lammel, S.; Tye, K. M.; Warden, M. R. (1 January 2014). "Progress in understanding mood disorders: optogenetic dissection of neural circuits". Genlar, miya va o'zini tutish. 13 (1): 38–51. doi:10.1111/gbb.12049. ISSN 1601-183X. PMID 23682971.
- ^ Delgado PL, Moreno FA (2000). "Role of norepinephrine in depression". J klinik psixiatriya. 61 Suppl 1: 5–12. PMID 10703757.
- ^ Ruhe, HG; Mason, NS; Schene, AH (2007). "Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies". Molekulyar psixiatriya. 12 (4): 331–359. doi:10.1038/sj.mp.4001949. PMID 17389902.
- ^ Meyer JH, Ginovart N, Boovariwala A, et al. (2006 yil noyabr). "Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression". Umumiy psixiatriya arxivi. 63 (11): 1209–16. doi:10.1001 / archpsyc.63.11.1209. PMID 17088501.
- ^ Huang SY, Lin MT, Lin WW, Huang CC, Shy MJ, Lu RB (19 December 2007). "Monoamin oksidaza A (MAOA) polimorfizmlari assotsiatsiyasi va Xan xitoy populyatsiyasidagi asosiy depressiv kasalliklarning klinik kichik guruhlari". World Journal of Biological Psychiatry. 10 (4 Pt 2): 544-51. doi:10.1080/15622970701816506. PMID 19224413.
- ^ Yu YW, Tsay SJ, Xong CJ, Chen TJ, Chen MC, Yang CW (sentyabr 2005). "Monoamin oksidaza gen depressiv polimorfizmini katta depressiya buzilishi va antidepressant reaktsiyasi bilan assotsiatsiyasini o'rganish". Nöropsikofarmakologiya. 30 (9): 1719–23. doi:10.1038 / sj.npp.1300785. PMID 15956990.
- ^ Cicchetti D, Rogosch FA, Sturge-Apple ML (2007). "Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds". Dev. Psixopatol. 19 (4): 1161–80. doi:10.1017/S0954579407000600. PMID 17931441.
- ^ Castrén, E (2005). "Is mood chemistry?". Neuroscience-ning tabiat sharhlari. 6 (3): 241–46. doi:10.1038/nrn1629. PMID 15738959.
- ^ Hirschfeld RM (2000). "History and evolution of the monoamine hypothesis of depression". Klinik psixiatriya jurnali. 61 Suppl 6: 4–6. PMID 10775017.
- ^ Davis, Kenneth L.; va boshq., tahr. (2002). Neuropsychopharmacology : the fifth generation of progress : an official publication of the American College of Neuropsychopharmacology (5-nashr). Filadelfiya, Pa.: Lippincott Uilyams va Uilkins. pp. 1139–1163. ISBN 9780781728379.
- ^ Jacobsen, Jacob P. R.; Medvedev, Ivan O.; Caron, Marc G. (5 September 2012). "The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse". Qirollik jamiyatining falsafiy operatsiyalari B: Biologiya fanlari. 367 (1601): 2444–2459. doi:10.1098/rstb.2012.0109. ISSN 0962-8436. PMC 3405680. PMID 22826344.
- ^ Delgado PL, Moreno FA (2000). "Role of norepinephrine in depression". J klinik psixiatriya. 61 Suppl 1: 5–12. PMID 10703757.
- ^ Delgado PL (2000). "Depression: the case for a monoamine deficiency". Klinik psixiatriya jurnali. 61 Suppl 6: 7–11. PMID 10775018.
- ^ Andrews, Paul W.; Bharwani, Aadil; Lee, Kyuwon R.; Fox, Molly; Thomson, J. Anderson (1 April 2015). "Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response". Neyrologiya va biobehavioral sharhlar. 51: 164–188. doi:10.1016/j.neubiorev.2015.01.018. ISSN 1873-7528. PMID 25625874.
- ^ Lacasse, Jeffrey R.; Leo, Jonathan (8 November 2005). "Serotonin and Depression: A Disconnect between the Advertisements and the Scientific Literature". PLOS tibbiyoti. 2 (12): e392. doi:10.1371/journal.pmed.0020392. PMC 1277931. PMID 16268734.

- ^ Savitz, Jonathan; Drevets, Wayne (2013). "Neuroreceptor imaging in depression". Kasallikning neyrobiologiyasi. 52: 49–65. doi:10.1016/j.nbd.2012.06.001. PMID 22691454.
- ^ Gryglewski, G; Lanzenberger, R; Kranz, GS; Cumming, P (July 2014). "Meta-analysis of molecular imaging of serotonin transporters in major depression". Miya qon oqimi va metabolizm jurnali. 34 (7): 1096–103. doi:10.1038/jcbfm.2014.82. PMC 4083395. PMID 24802331.
- ^ Kambeitz, JP; Howes, OD (1 November 2015). "The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression". Affektiv buzilishlar jurnali. 186: 358–66. doi:10.1016/j.jad.2015.07.034. PMID 26281039.
- ^ Vang, L; Chjou, S; Chu, D; Vang, X; Fang, L; Zhong, J; Mao, Q; Quyosh, L; Gong, X; Xia, J; Lian, B; Xie, P (13 September 2016). "Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies". BMC psixiatriyasi. 16 (1): 319. doi:10.1186/s12888-016-1025-0. PMC 5022168. PMID 27623971.
- ^ Li, Z; U, Y; Tang, J; Zong, X; Hu, M; Chen, X (15 March 2015). "Molecular imaging of striatal dopamine transporters in major depression—a meta-analysis". Affektiv buzilishlar jurnali. 174: 137–43. doi:10.1016/j.jad.2014.11.045. PMID 25497470.
- ^ Bourke, Cecilia; Douglas, Katie; Porter, Richard (1 August 2010). "Processing of facial emotion expression in major depression: a review". Avstraliya va Yangi Zelandiya psixiatriya jurnali. 44 (8): 681–696. doi:10.3109/00048674.2010.496359. ISSN 1440-1614. PMID 20636189.
- ^ Dalili, M. N.; Penton-Voak, I. S.; Harmer, C. J.; Munafò, M. R. (7 December 2016). "Meta-analysis of emotion recognition deficits in major depressive disorder". Psixologik tibbiyot. 45 (6): 1135–1144. doi:10.1017/S0033291714002591. ISSN 0033-2917. PMC 4712476. PMID 25395075.
- ^ Groenewold, Nynke A.; Opmeer, Esther M.; de Jonge, Peter; Aleman, Andre; Costafreda, Sergi G. (1 February 2013). "Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies". Neyrologiya va biobehavioral sharhlar. 37 (2): 152–163. doi:10.1016/j.neubiorev.2012.11.015. ISSN 1873-7528. PMID 23206667.
- ^ Müller, VI; Cieslik, EC; Serbanescu, I; Laird, AR; Fox, PT; Eickhoff, SB (1 January 2017). "Altered Brain Activity in Unipolar Depression Revisited: Meta-analyses of Neuroimaging Studies". JAMA psixiatriyasi. 74 (1): 47–55. doi:10.1001/jamapsychiatry.2016.2783. PMC 5293141. PMID 27829086.
- ^ Harmer, C. J.; Gudvin, G. M .; Cowen, P. J. (31 July 2009). "Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action". Britaniya psixiatriya jurnali. 195 (2): 102–108. doi:10.1192/bjp.bp.108.051193. PMID 19648538.
- ^ Delaveau, P; Jabourian, M; Lemogne, C; Guionnet, S; Bergouignan, L; Fossati, P (April 2011). "Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies". Affektiv buzilishlar jurnali. 130 (1–2): 66–74. doi:10.1016/j.jad.2010.09.032. PMID 21030092.
- ^ Pringle, A; Harmer, CJ (December 2015). "The effects of drugs on human models of emotional processing: an account of antidepressant drug treatment". Klinik nevrologiya sohasidagi suhbatlar. 17 (4): 477–87. PMC 4734885. PMID 26869848.
- ^ a b v Hamilton, J. Paul; Etkin, Amit; Furman, Daniella J.; Lemus, Maria G.; Johnson, Rebecca F.; Gotlib, Ian H. (1 July 2012). "Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data". Amerika psixiatriya jurnali. 169 (7): 693–703. doi:10.1176/appi.ajp.2012.11071105. ISSN 1535-7228. PMID 22535198.
- ^ Drevets, WC; Narx, JL; Furey, ML (September 2008). "Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression". Miyaning tuzilishi va funktsiyasi. 213 (1–2): 93–118. doi:10.1007/s00429-008-0189-x. PMC 2522333. PMID 18704495.
- ^ Drevets, WC (April 2001). "Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders". Neyrobiologiyaning hozirgi fikri. 11 (2): 240–9. doi:10.1016/S0959-4388(00)00203-8. PMID 11301246.
- ^ Mayberg, Helen (1 August 1997). "Limbic-cortical dysregulation: a proposed model of depression". Nöropsikiyatriya va klinik nevrologiya jurnali. 9 (3): 471–481. doi:10.1176/jnp.9.3.471. ISSN 0895-0172. PMID 9276848.
- ^ a b Graham, Julia; Salimi-Xorshidi, G'ulamreza; Hagan, Cindy; Walsh, Nicholas; Gudyer, Yan; Lennox, Belinda; Suckling, John (1 November 2013). "Meta-analytic evidence for neuroimaging models of depression: State or trait?". Affektiv buzilishlar jurnali. 151 (2): 423–431. doi:10.1016/j.jad.2013.07.002. PMID 23890584.
- ^ Anticevic, A; Schleifer, C; Youngsun, TC (December 2015). "Emotional and cognitive dysregulation in schizophrenia and depression: understanding common and distinct behavioral and neural mechanisms". Klinik nevrologiya sohasidagi suhbatlar. 17 (4): 421–34. PMC 4734880. PMID 26869843.
- ^ Rømer Thomsen, K; Whybrow, PC; Kringelbach, ML (2015). "Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain". Xulq-atvor nevrologiyasidagi chegaralar. 9: 49. doi:10.3389/fnbeh.2015.00049. PMC 4356228. PMID 25814941.
- ^ Treadway, MT; Zald, DH (January 2011). "Reconsidering anhedonia in depression: lessons from translational neuroscience". Neyrologiya va biobehavioral sharhlar. 35 (3): 537–55. doi:10.1016/j.neubiorev.2010.06.006. PMC 3005986. PMID 20603146.
- ^ a b v Sternat T, Katzman MA (1 January 2016). "Neurobiology of hedonic tone: the relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse". Nöropsikiyatrik kasallik va davolash. 12: 2149–64. doi:10.2147/NDT.S111818. PMC 5003599. PMID 27601909.
- ^ Russo, SJ; Nestler, EJ (September 2013). "The brain reward circuitry in mood disorders". Tabiat sharhlari. Nevrologiya. 14 (9): 609–25. doi:10.1038/nrn3381. PMC 3867253. PMID 23942470.
- ^ Rolls, ET (September 2016). "A non-reward attractor theory of depression" (PDF). Neyrologiya va biobehavioral sharhlar. 68: 47–58. doi:10.1016/j.neubiorev.2016.05.007. PMID 27181908.
- ^ Miller, CH; Hamilton, JP; Sacchet, MD; Gotlib, IH (October 2015). "Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth". JAMA psixiatriyasi. 72 (10): 1045–53. doi:10.1001/jamapsychiatry.2015.1376. PMID 26332700.
- ^ Graham, J; Salimi-Khorshidi, G; Hagan, C; Walsh, N; Goodyer, I; Lennox, B; Suckling, J (November 2013). "Meta-analytic evidence for neuroimaging models of depression: state or trait?". Affektiv buzilishlar jurnali. 151 (2): 423–31. doi:10.1016/j.jad.2013.07.002. PMID 23890584.
- ^ Drevets, WC; Savitz, J; Trimble, M (August 2008). "The subgenual anterior cingulate cortex in mood disorders". CNS spektrlari. 13 (8): 663–81. doi:10.1017/S1092852900013754. PMC 2729429. PMID 18704022.
- ^ a b Lammel, S; Tye, KM; Warden, MR (January 2014). "Progress in understanding mood disorders: optogenetic dissection of neural circuits". Genlar, miya va o'zini tutish. 13 (1): 38–51. doi:10.1111/gbb.12049. PMID 23682971.
- ^ Groenewold, NA; Opmeer, EM; de Jonge, P; Aleman, A; Costafreda, SG (February 2013). "Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies". Neyrologiya va biobehavioral sharhlar. 37 (2): 152–63. doi:10.1016/j.neubiorev.2012.11.015. PMID 23206667.
- ^ Drevets, WC; Savitz, J; Trimble, M (August 2008). "The subgenual anterior cingulate cortex in mood disorders". CNS spektrlari. 13 (8): 663–81. doi:10.1017/S1092852900013754. PMC 2729429. PMID 18704022.
Together, these data suggest the hypothesis that dysfunction of the sgACC results in understimulation of parasympathetic tone in mood disorders.
- ^ Rive, MM; van Rooijen, G; Veltman, DJ; Phillips, ML; Schene, AH; Ruhé, HG (December 2013). "Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies". Neyrologiya va biobehavioral sharhlar. 37 (10 Pt 2): 2529–53. doi:10.1016/j.neubiorev.2013.07.018. PMID 23928089.
- ^ Dunlop, BW; Mayberg, HS (December 2014). "Neuroimaging-based biomarkers for treatment selection in major depressive disorder". Klinik nevrologiya sohasidagi suhbatlar. 16 (4): 479–90. PMC 4336918. PMID 25733953.
- ^ Wise, T; Radua, J; Via, E; Cardoner, N; Abe, O; Adams, TM; Amico, F; Cheng, Y; Cole, JH; de Azevedo Marques Périco, C; Dickstein, DP; Farrow, TFD; Frodl, T; Vagner, G; Gotlib, IH; Gruber, O; Ham, BJ; Job, DE; Kempton, MJ; Kim, MJ; Koolschijn, PCMP; Malhi, GS; Mataix-Cols, D; McIntosh, AM; Nugent, AC; O'Brien, JT; Pezzoli, S; Phillips, ML; Sachdev, PS; Salvadore, G; Selvaraj, S; Stanfield, AC; Thomas, AJ; van Tol, MJ; van der Wee, NJA; Veltman, DJ; Young, AH; Fu, CH; Cleare, AJ; Arnone, D (October 2017). "Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis". Molekulyar psixiatriya. 22 (10): 1455–1463. doi:10.1038/mp.2016.72. PMC 5622121. PMID 27217146.
- ^ Bora, E; Fornito, A; Pantelis, C; Yücel, M (April 2012). "Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies". Affektiv buzilishlar jurnali. 138 (1–2): 9–18. doi:10.1016/j.jad.2011.03.049. PMID 21511342.
- ^ Chjan, H; Li, L; Vu, M; Chen, Z; Hu, X; Chen, Y; Chju, H; Jia, Z; Gong, Q (January 2016). "Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies". Neyrologiya va biobehavioral sharhlar. 60: 43–50. doi:10.1016/j.neubiorev.2015.10.011. PMID 26592799.
- ^ Zhao, YJ; Du, MY; Huang, XQ; Lui, S; Chen, ZQ; Liu, J; Luo, Y; Wang, XL; Kemp, GJ; Gong, QY (October 2014). "Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis". Psixologik tibbiyot. 44 (14): 2927–37. doi:10.1017/S0033291714000518. PMID 25065859.
- ^ Sacher, J; Neumann, J; Fünfstück, T; Soliman, A; Villringer, A; Schroeter, ML (October 2012). "Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder". Affektiv buzilishlar jurnali. 140 (2): 142–8. doi:10.1016/j.jad.2011.08.001. PMID 21890211.
- ^ Arnone, D; Job, D; Selvaraj, S; Abe, O; Amico, F; Cheng, Y; Colloby, SJ; O'Brien, JT; Frodl, T; Gotlib, IH; Ham, BJ; Kim, MJ; Koolschijn, PC; Périco, CA; Salvadore, G; Thomas, AJ; Van Tol, MJ; van der Wee, NJ; Veltman, DJ; Vagner, G; McIntosh, AM (April 2016). "Computational meta-analysis of statistical parametric maps in major depression". Insonning miya xaritasini tuzish. 37 (4): 1393–404. doi:10.1002/hbm.23108. PMC 6867585. PMID 26854015.
- ^ Schmaal, L; Hibar, DP; Sämann, PG; Hall, GB; Baune, BT; Jahanshad, N; Cheung, JW; van Erp, TGM; Bos, D; Ikrom, MA; Vernooij, MV; Nissen, VJ; Tiemeier, H; Xofman, A; Wittfeld, K; Grabe, HJ; Janowitz, D; Bülow, R; Selonke, M; Völzke, H; Grotegerd, D; Dannlowski, U; Arolt, V; Opel, N; Heindel, W; Kugel, H; Hoehn, D; Czisch, M; Couvy-Duchesne, B; Rentería, ME; Strike, LT; Wright, MJ; Mills, NT; de Zubicaray, GI; McMahon, KL; Medland, SE; Martin, NG; Gillespie, NA; Goya-Maldonado, R; Gruber, O; Krämer, B; Hatton, SN; Lagopoulos, J; Hickie, IB; Frodl, T; Carballedo, A; Frey, EM; van Velzen, LS; Penninx, BWJH; van Tol, MJ; van der Wee, NJ; Davey, CG; Harrison, BJ; Mwangi, B; Cao, B; Soares, JK; Veer, IM; Walter, H; Schoepf, D; Zurowski, B; Konrad, C; Schramm, E; Normann, C; Schnell, K; Sacchet, MD; Gotlib, IH; MacQueen, GM; Godlewska, BR; Nickson, T; McIntosh, AM; Papmeyer, M; Whalley, HC; Hall, J; Sussmann, JE; Li, M; Walter, M; Aftanas, L; Brack, I; Bokhan, NA; Thompson, PM; Veltman, DJ (June 2017). "Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group". Molekulyar psixiatriya. 22 (6): 900–909. doi:10.1038/mp.2016.60. PMC 5444023. PMID 27137745.
- ^ Shmaal, L; Veltman, DJ; van Erp, TG; Sämann, PG; Frodl, T; Jahonshad, N; Loehrer, E; Tiemeier, H; Xofman, A; Nissen, VJ; Vernooij, MV; Ikrom, MA; Wittfeld, K; Grab, XJ; Blok, A; Hegenscheid, K; Volske, H; Xayn, D; Cisch, M; Lagopulos, J; Xatton, SN; Xiki, IB; Goya-Maldonado, R; Kremer, B; Gruber, O; Kuvi-Dyuzne, B; Renteriya, ME; Strike, LT; Mills, NT; de Zubikaray, GI; McMahon, KL; Medland, SE; Martin, NG; Gillespi, NA; Rayt, MJ; Hall, GB; MacQueen, GM; Frey, EM; Karballedo, A; van Velzen, LS; van Tol, MJ; van der Vi, NJ; Veer, IM; Uolter, H; Shnell, K; Shramm, E; Normann, C; Shoepf, D; Konrad, C; Zurovskiy, B; Nikkson, T; McIntosh, AM; Papmeyer, M; Whalley, HC; Sussmann, JE; Godlewska, BR; Koven, PJ; Fischer, FH; Rose, M; Penninx, BW; Tompson, bosh vazir; Hibar, DP (iyun 2016). "Katta depressiya buzilishida subkortikal miya o'zgarishlari: ENIGMA Major Depressiv Disorder ishchi guruhi xulosalari". Molekulyar psixiatriya. 21 (6): 806–12. doi:10.1038 / mp.2015.69. PMC 4879183. PMID 26122586.
- ^ a b v d Chen, G; Xu, X; Li, L; Xuang, X; Lui, S; Kuang, V; Ai, H; Bi, F; Gu, Z; Gong, Q (2016 yil 24-fevral). "Katta depressiya buzilishida oq modda me'morchiligining buzilishi: traktga asoslangan fazoviy statistika bilan diffuziya tensorli tasvirining meta-tahlili". Ilmiy ma'ruzalar. 6: 21825. Bibcode:2016 yil NatSR ... 621825C. doi:10.1038 / srep21825. PMC 4764827. PMID 26906716.
- ^ a b v d Chen, G; Guo, Y; Chju, H; Kuang, V; Bi, F; Ai, H; Gu, Z; Xuang, X; Lui, S; Gong, Q (2017 yil 2-iyun). "Birinchi epizoddagi oq modda mikroarxitekturasining ichki buzilishi, dori-darmonli asosiy depressiv buzuqlik: diffuziya tensorini tasvirlashning vokselga asoslangan meta-tahlili". Neyro-psixofarmakologiya va biologik psixiatriyadagi taraqqiyot. 76: 179–187. doi:10.1016 / j.pnpbp.2017.03.011. PMID 28336497.
- ^ a b v d Tszyan, J; Chjao, YJ; Xu, XY; Du, mening; Chen, ZQ; Vu, M; Li, KM; Zhu, HY; Kumar, P; Gong, QY (may, 2017). "Majburiy depressiya buzilishi bo'lgan dori-darmonsiz bemorlarda miyaning mikroyapısal anormalliklari: diffuziya tensorini ko'rishning tizimli tekshiruvi va meta-tahlili". Psixiatriya va nevrologiya jurnali. 42 (3): 150–163. doi:10.1503 / jpn.150341. PMC 5403660. PMID 27780031.
- ^ a b Dono, T; Radua, J; Nortje, G; Cleare, AJ; Yosh, AH; Arnone, D (2016 yil 15-fevral). "Katta depressiya va bipolyar buzuqlikdagi strukturaviy uzilishning Voxel asosidagi meta-analitik dalillari". Biologik psixiatriya. 79 (4): 293–302. doi:10.1016 / j.biopsych.2015.03.004. PMID 25891219.
- ^ Chjou, M; Xu, X; Lu, L; Chjan, L; Chen, L; Gong, Q; Huang, X (2017 yil 3-aprel). "Katta depressiya buzilishi bo'lgan kattalardagi dam olish holatidagi ichki miya faoliyati: meta-tahlil". Neyro-psixofarmakologiya va biologik psixiatriyadagi taraqqiyot. 75: 157–164. doi:10.1016 / j.pnpbp.2017.02.001. PMID 28174129.
- ^ Li, V; Chen, Z; Vu, M; Chju, H; Gu, L; Chjao, Y; Kuang, V; Bi, F; Kemp, GJ; Gong, Q (2017 yil 1 mart). "Miya qon oqimining xarakteristikasi va asosiy depressiv buzuqlikdagi past chastotali dalgalanmalar amplitudasi: multimodal meta-tahlil". Affektiv buzilishlar jurnali. 210: 303–311. doi:10.1016 / j.jad.2016.12.032. PMID 28068619.
- ^ Kyun, S; Gallinat, J (mart, 2013). "Shizofreniya va katta depressiya holatidagi miyaning dam olish holati: miqdoriy meta-tahlil". Shizofreniya byulleteni. 39 (2): 358–65. doi:10.1093 / schbul / sbr151. PMC 3576173. PMID 22080493.
- ^ a b Fitsjerald, PB; Laird, AR; Maller, J; Daskalakis, ZJ (iyun 2008). "Depressiyada miya faollashishi o'zgarishlarini meta-analitik o'rganish". Insonning miya xaritasini tuzish. 29 (6): 683–95. doi:10.1002 / hbm.20426. PMC 2873772. PMID 17598168.
- ^ Karlson, Nil R. (2012). Xulq-atvor fiziologiyasi kitoblari La Carte nashri (11-nashr). Boston: Pearson kolleji div. ISBN 978-0-205-23981-8.
- ^ a b Miller, Kris X.; Xemilton, J. Pol; Sakkhet, Metyu D.; Gotlib, Yan H. (1 oktyabr 2015). "Yoshlarda asosiy depressiya buzilishining funktsional neyroimaging meta-tahlili". JAMA psixiatriyasi. 72 (10): 1045–1053. doi:10.1001 / jamapsychiatry.2015.1376. ISSN 2168-6238. PMID 26332700.
- ^ Vessa, Miyele; Lois, Giannis (2016 yil 30-noyabr). "Katta depressiyada psixofarmakologik davolanishning miyaning funktsional ta'siri: ta'sirchan ishlov berishning asabiy aylanishiga e'tibor". Hozirgi neyrofarmakologiya. 13 (4): 466–479. doi:10.2174 / 1570159X13666150416224801. ISSN 1570-159X. PMC 4790403. PMID 26412066.
- ^ Outhred, Tim; Xokshed, Bretaniy E.; Vager, Tor D.; Das, Prita; Malhi, Jin S.; Kemp, Endryu H. (2013 yil 1 sentyabr). "Selektiv serotoninni qaytarib olish inhibitörlerinin noradrenalinni qaytarib olish inhibitörlerine qarshi hissiyotlarni qayta ishlashga o'tkir asabiy ta'siri: davolashning differentsial samaradorligiga ta'siri" (PDF). Neyrologiya va biobehavioral sharhlar. 37 (8): 1786–1800. doi:10.1016 / j.neubiorev.2013.07.010. ISSN 1873-7528. PMID 23886514.
- ^ Xemilton, J. Pol; Siemer, Matias; Gotlib, Yan H. (8 sentyabr 2009). "Asosiy depressiv buzuqlikdagi amigdala hajmi: magnit-rezonans tomografiya bo'yicha meta-tahlil". Molekulyar psixiatriya. 13 (11): 993–1000. doi:10.1038 / mp.2008.57. ISSN 1359-4184. PMC 2739676. PMID 18504424.
- ^ Palmer, Syuzan M.; Krewter, Sheila G.; Carey, Leeanne M. (2015 yil 14-yanvar). "Klinik depressiyada miya faoliyati o'zgarishini meta-tahlili". Inson nevrologiyasidagi chegaralar. 8: 1045. doi:10.3389 / fnhum.2014.01045. ISSN 1662-5161. PMC 4294131. PMID 25642179.
- ^ Fitsjerald, Pol B.; Laird, Angela R.; Maller, Jerom; Daskalakis, Zafiris J. (2016 yil 5-dekabr). "Depressiyada miya faollashishi o'zgarishini meta-analitik o'rganish". Insonning miya xaritasini tuzish. 29 (6): 683–695. doi:10.1002 / hbm.20426. ISSN 1065-9471. PMC 2873772. PMID 17598168.
- ^ Koul, Jeyms; Kostafreda, Sergi G.; Makguffin, Piter; Fu, Sintiya H. Y. (2011 yil 1-noyabr). "Birinchi epizod depressiyasida gipokampal atrofiya: magnit-rezonans tomografiya tadqiqotlarining meta-tahlili". Affektiv buzilishlar jurnali. 134 (1–3): 483–487. doi:10.1016 / j.jad.2011.05.057. ISSN 1573-2517. PMID 21745692.
- ^ Videbech, Poul; Ravnkilde, Barbara (2004 yil 1-noyabr). "Gipokampal hajmi va depressiyasi: MRI tadqiqotlarining meta-tahlili". Amerika psixiatriya jurnali. 161 (11): 1957–1966. doi:10.1176 / appi.ajp.161.11.1957. ISSN 0002-953X. PMID 15514393.
- ^ Mahar, men; Bambiko, FR; Mexavar, N; Nobrega, JN (2014 yil yanvar). "Depressiya va antidepressant ta'siriga nisbatan stress, serotonin va hipokampal neyrogenez". Neyrologiya va biobehavioral sharhlar. 38: 173–92. doi:10.1016 / j.neubiorev.2013.11.009. PMID 24300695.
- ^ Willner, P; Scheel-Krüger, J; Belzung, S (2013 yil dekabr). "Depressiya va antidepressant ta'sirining neyrobiologiyasi". Neyrologiya va biobehavioral sharhlar. 37 (10 Pt 1): 2331-71. doi:10.1016 / j.neubiorev.2012.12.007. PMID 23261405.
- ^ Pariante CM, Lightman SL (sentyabr 2008). "Katta depressiyadagi HPA o'qi: klassik nazariyalar va yangi o'zgarishlar". Neurosci tendentsiyalari. 31 (9): 464–468. doi:10.1016 / j.tins.2008.06.006. PMID 18675469.
- ^ Belvederi Murri, Martino; Pariante, Karmin; Mondelli, Valeriya; Masotti, Mattiya; Atti, Anna Rita; Melakva, Zefiro; Antonioli, Marko; Gio, Lusio; Menchetti, Marko; Zanetidou, Stamatula; Innamorati, Marko; Amore, Mario (2014 yil 1 mart). "HPA o'qi va depressiyada qarish: sistematik tahlil va meta-tahlil". Psixonuroendokrinologiya. 41: 46–62. doi:10.1016 / j.psyneuen.2013.12.12.004. ISSN 1873-3360. PMID 24495607.
- ^ Juruena, Mario F. (2014 yil 1 sentyabr). "Erta hayotdagi stress va HPA o'qi kattalar uchun takroriy depressiyani keltirib chiqaradi". Epilepsiya va o'zini tutish. 38: 148–159. doi:10.1016 / j.yebeh.2013.10.020. ISSN 1525-5069. PMID 24269030.
- ^ Xeym, Kristin; Nyuport, D. Jefri; Mletzko, Tanja; Miller, Endryu X.; Nemeroff, Charlz B. (2008 yil 1-avgust). "Bolalik travması va depressiya o'rtasidagi bog'liqlik: odamlarda HPA o'qi bo'yicha tushunchalar". Psixonuroendokrinologiya. 33 (6): 693–710. doi:10.1016 / j.psyneuen.2008.03.008. ISSN 0306-4530. PMID 18602762.
- ^ Arana, G. V.; Baldessarini, R. J .; Ornsteen, M. (1985 yil 1-dekabr). "Psixiatriyada diagnostika va prognoz uchun deksametazonni bostirish testi. Sharh va sharh". Umumiy psixiatriya arxivi. 42 (12): 1193–1204. doi:10.1001 / archpsyc.1985.01790350067012. ISSN 0003-990X. PMID 3000317.
- ^ Varghese, Femina P.; Braun, E. Shervud (2001 yil 1-yanvar). "Asosiy depressiv kasallikdagi gipotalamus-gipofiz-buyrak usti o'qi: birlamchi tibbiy yordam ko'rsatuvchi shifokorlar uchun qisqacha ma'lumot". Klinik psixiatriya jurnaliga birlamchi tibbiy yordam. 3 (4): 151–155. doi:10.4088 / pcc.v03n0401. ISSN 1523-5998. PMC 181180. PMID 15014598.
- ^ Gilbertson, M. V.; Shenton, M. E.; Ciszewski, A .; Kasay K .; Lasko, N. B.; Orr, S. P.; Pitman, R. K. (2002). "Gipokampalning kichik hajmi psixologik travmaya patologik zaiflikni bashorat qiladi". Tabiat nevrologiyasi. 5 (11): 1242–7. doi:10.1038 / nn958. PMC 2819093. PMID 12379862.
- ^ Vitilingam, Meena; Vermetten, Erik; Anderson, Jorj M.; Luckenbaugh, Devid; Anderson, Erik R.; Qor, Jozef; Stayb, Lourens X.; Charney, Dennis S.; Bremner, J. Duglas (2004 yil 15-iyul). "Katta depressiya buzilishida hipokampal hajmi, xotirasi va kortizol holati: davolash ta'siri". Biologik psixiatriya. 56 (2): 101–112. doi:10.1016 / j.biopsych.2004.04.002. ISSN 0006-3223. PMID 15231442.
- ^ a b v Krishnan, V; Nestler, EJ (2011). Depressiyaning hayvonot modellari: molekulyar istiqbollar. Xulq-atvor nevrologiyasining dolzarb mavzulari. 7. 121-47 betlar. doi:10.1007/7854_2010_108. ISBN 978-3-642-19702-4. PMC 3270071. PMID 21225412.
- ^ Belzung, C; Lemoine, M (2011 yil 7-noyabr). "Psixiatrik kasalliklarning hayvon modellari uchun amal qilish mezonlari: anksiyete va depressiyaga e'tibor bering". Kayfiyat va bezovtalik buzilishi biologiyasi. 1 (1): 9. doi:10.1186/2045-5380-1-9. PMC 3384226. PMID 22738250.
- ^ a b Alkantara, Lionna F.; Maqtov, Erik M.; Bolanos-Guzman, Karlos A. (2018). "26. Kayfiyatni buzadigan hayvonlarning modellari". Charneyda, Dennis; Sklar, Pamela; Nestler, Erik; Buxbaum, Jozef (tahrir). Charney & Nestlerning ruhiy kasallik neyrobiologiyasi (5-nashr). Nyu-York: Oksford universiteti matbuoti. 329–333 betlar.
- ^ Yan, XK; Cao, X; Das, M; Zhu, XH; Gao, TM (avgust, 2010). "Hayvonlarning ruhiy tushkunlik modellari". Neuroscience byulleteni. 26 (4): 327–37. doi:10.1007 / s12264-010-0323-7. PMC 5552573. PMID 20651815.
- ^ Chex, B; Fuks, E; Viborg, O; Simon, M (2016 yil 4-yanvar). "Katta depressiyaning hayvon modellari va ularning klinik oqibatlari". Neyro-psixofarmakologiya va biologik psixiatriyadagi taraqqiyot. 64: 293–310. doi:10.1016 / j.pnpbp.2015.04.004. PMID 25891248.
- ^ a b v Yang, Y; Vang, H; Xu, J; Xu, H (2018 yil fevral). "Depressiya patofizyologiyasida lateral habenula". Neyrobiologiyaning hozirgi fikri. 48: 90–96. doi:10.1016 / j.conb.2017.10.024. PMID 29175713.
- ^ Proulx, CD; Hikosaka, O; Malinov, R (sentyabr 2014). "Oddiy va depressiv xatti-harakatlarda lateral habenula tomonidan mukofotni qayta ishlash". Tabiat nevrologiyasi. 17 (9): 1146–52. doi:10.1038 / nn.3779. PMC 4305435. PMID 25157511.
- ^ Belujon, P; Grace, AA (2017 yil 1-dekabr). "Asosiy depressiv kasalliklarda dopamin tizimining regulyatsiyasi". Xalqaro neyropsikofarmakologiya jurnali. 20 (12): 1036–1046. doi:10.1093 / ijnp / pyx056. PMC 5716179. PMID 29106542.
- ^ a b Knowlend, D; Lim, BK (2018 yil 5-yanvar). "Depressiv xatti-harakatlarning elektron asoslari: mukofot tizimining roli va undan tashqarida". Farmakologiya Biokimyo va o'zini tutish. 174: 42–52. doi:10.1016 / j.pbb.2017.12.010. PMC 6340396. PMID 29309799.
- ^ Heller, AS (2016). "Depressiyadagi kortikal-subkortikal o'zaro ta'sirlar: hayvonlar modelidan inson psixopatologiyasigacha". Tizimlar nevrologiyasidagi chegaralar. 10: 20. doi:10.3389 / fnsys.2016.00020. PMC 4780432. PMID 27013988.
- ^ Kristofer Pittenger; Ronald S Duman (2008). "Stress, depressiya va neyroplastiklik: mexanizmlarning yaqinlashuvi". Nöropsikofarmakologiya. 33 (1): 88–109. doi:10.1038 / sj.npp.1301574. PMID 17851537.
- ^ Brunoni, Andre Russkiy; Lopes, Mariana; Fregni, Felipe (2008 yil 1-dekabr). "Katta depressiya va BDNF darajalari bo'yicha klinik tadqiqotlarni muntazam ravishda qayta ko'rib chiqish va meta-tahlil: depressiyada neyroplastikaning roliga ta'siri". Xalqaro neyropsikofarmakologiya jurnali. 11 (8): 1169–1180. doi:10.1017 / S1461145708009309. ISSN 1461-1457. PMID 18752720.
- ^ Serafini, Janluka (2012 yil 22-iyun). "Neyroplastiklik va katta depressiya, zamonaviy antidepressant dorilarning ahamiyati". Butunjahon psixiatriya jurnali. 2 (3): 49–57. doi:10.5498 / wjp.v2.i3.49. ISSN 2220-3206. PMC 3782176. PMID 24175168.
- ^ Krishnadas, Rajeev; Cavanagh, Jonathan (2012 yil 1-may). "Depressiya: yallig'lanish kasalligi?". Nevrologiya, neyroxirurgiya va psixiatriya jurnali. 83 (5): 495–502. doi:10.1136 / jnnp-2011-301779. ISSN 1468-330X. PMID 22423117.
- ^ Patel, Amisha (2013 yil 1 sentyabr). "Ko'rib chiqish: depressiyada yallig'lanishning roli". Psixiatriya Danubina. 25 Qo'shimcha 2: S216-223. ISSN 0353-5053. PMID 23995180.
- ^ Dowlati, Yekta; Herrmann, Natan; Swardfager, Valter; Liu, Xelena; Sham, Loren; Reym, Yelis K.; Lanktot, Krista L. (2010 yil 1 mart). "Katta depressiyada sitokinlarning meta-tahlili". Biologik psixiatriya. 67 (5): 446–457. doi:10.1016 / j.biopsich.2009.09.033. ISSN 1873-2402. PMID 20015486.
- ^ Dantzer, Robert; O'Konnor, Jeyson S.; Freund, Gregori G.; Jonson, Rodni V.; Kelley, Keyt W. (3 dekabr 2016). "Yallig'lanishdan kasallik va depressiyaga: immunitet tizimi miyani bo'ysundirganda". Neuroscience-ning tabiat sharhlari. 9 (1): 46–56. doi:10.1038 / nrn2297. ISSN 1471-003X. PMC 2919277. PMID 18073775.
- ^ Xilz, Sara A .; Beyker, Amanda L.; de Malmanche, Teo; Attia, Jon (2012 yil 1 oktyabr). "Depressiyaga chalingan va tushkun bo'lmagan odamlar o'rtasidagi IL-6 va IL-10 tafovutlarining meta-tahlili: heterojenlik sabablarini o'rganish". Miya, o'zini tutish va immunitet. 26 (7): 1180–1188. doi:10.1016 / j.bbi.2012.06.001. ISSN 1090-2139. PMID 22687336.
- ^ Xoren, M. Brayant; Lamkin, Donald M.; Suls, Jerri (2009 yil 1-fevral). "C-reaktiv oqsil, IL-1 va IL-6 bilan depressiyaning assotsiatsiyasi: meta-tahlil". Psixosomatik tibbiyot. 71 (2): 171–186. doi:10.1097 / PSY.0b013e3181907c1b. ISSN 1534-7796. PMID 19188531.
- ^ Maes, Maykl (2011 yil 29 aprel). "Depressiya yallig'lanish kasalligi, ammo hujayralar vositasida immunitet faollashishi depressiyaning asosiy tarkibiy qismidir". Neyro-psixofarmakologiya va biologik psixiatriyadagi taraqqiyot. 35 (3): 664–675. doi:10.1016 / j.pnpbp.2010.06.014. ISSN 1878-4216. PMID 20599581.
- ^ Berk, Maykl; Uilyams, Lana J; Jacka, Felice N; O'Nil, Adrien; Pasko, Julie A; Moylan, Stiven; Allen, Nikolay B; Styuart, Amanda L; Xeyli, Ami S; Byorn, Mishel L; Maes, Maykl (2013 yil 12 sentyabr). "Demak, depressiya yallig'lanish kasalligi, ammo yallig'lanish qayerdan paydo bo'ladi?". BMC tibbiyoti. 11: 200. doi:10.1186/1741-7015-11-200. ISSN 1741-7015. PMC 3846682. PMID 24228900.
- ^ Leonard, Brayan; Maes, Maykl (2012 yil 1-fevral). "Hujayra vositachiligida immunitet faollashishi, yallig'lanish va oksidlanish va nitrozativ stress yo'llari, ularning davomi va qo'shni moddalari bir qutbli depressiya patofizyologiyasida qanday rol o'ynashi haqida mexanik tushuntirishlar". Neyrologiya va biobehavioral sharhlar. 36 (2): 764–785. doi:10.1016 / j.neubiorev.2011.12.005. ISSN 1873-7528. PMID 22197082.
- ^ Raedler, Tomas J. (2011 yil 1-noyabr). "Katta depressiv buzuqlikdagi yallig'lanish mexanizmlari". Psixiatriyadagi hozirgi fikr. 24 (6): 519–525. doi:10.1097 / YCO.0b013e32834b9db6. ISSN 1473-6578. PMID 21897249.
- ^ Kyler, Ole; Benros, Maykl E.; Nordentoft, Merete; Farkouh, Maykl E.; Iyengar, Rupa L.; Mors, Ole; Krogh, Jesper (2014 yil 1-dekabr). "Yallig'lanishga qarshi davolanishning depressiya, depressiv alomatlar va nojo'ya ta'sirlarga ta'siri: randomizatsiyalangan klinik tekshiruvlarning tizimli tekshiruvi va meta-tahlili". JAMA psixiatriyasi. 71 (12): 1381–1391. doi:10.1001 / jamapsychiatry.2014.1611. ISSN 2168-6238. PMID 25322082.
- ^ Medina, Jonna L.; Jakart, Jolen; Smits, Jasper A. J. (2015). "Depressiya uchun mashqlar retseptini optimallashtirish: javob biomarkerlarini qidirish". Psixologiyaning hozirgi fikri. 4: 43–47. doi:10.1016 / j.copsyc.2015.02.003. ISSN 2352-250X. PMC 4545504. PMID 26309904.
- ^ Parker GB, Brotchie H, Grem RK (2017 yil yanvar). "D vitamini va depressiya". J buzuqlikka ta'sir qiladi. 208: 56–61. doi:10.1016 / j.jad.2016.08.082. PMID 27750060.
- ^ Cenik B, Cenik C, Snyder MP, Brown ES (2017). "Populyatsion kogortada plazmadagi sterollar va depressiv simptomlarning og'irligi". PLOS One. 12 (9): e0184382. Bibcode:2017PLoSO..1284382C. doi:10.1371 / journal.pone.0184382. PMC 5590924. PMID 28886149.
- ^ Qora, Ketrin N.; Bot, Mariska; Sheffer, Piter G.; Kujpers, Pim; Penninx, Brenda W. J. H. (1 yanvar 2015). "Depressiya oksidlovchi stressning kuchayishi bilan bog'liqmi? Muntazam ko'rib chiqish va meta-tahlil". Psixonuroendokrinologiya. 51: 164–175. doi:10.1016 / j.psyneuen.2014.09.025. ISSN 1873-3360. PMID 25462890.
- ^ Liu, Tao; Chjun, Shuming; Liao, Xiaoxiao; Chen, Dzyan; U, Tingting; Lay, Shunkay; Jia, Yanbin (2015 yil 1-yanvar). "Depressiyada oksidlovchi stress belgilarining meta-tahlili". PLOS One. 10 (10): e0138904. Bibcode:2015PLoSO..1038904L. doi:10.1371 / journal.pone.0138904. ISSN 1932-6203. PMC 4596519. PMID 26445247.
- ^ Raza MU, Tufan T, Vang Y, Hill S, Chju MY (avgust 2016). "Asosiy psixiatrik kasalliklarda DNKning shikastlanishi". Neyrotoks Res. 30 (2): 251–67. doi:10.1007 / s12640-016-9621-9. PMC 4947450. PMID 27126805.
- ^ Liu, Tao; Chjun, Shuming; Liao, Xiaoxiao; Chen, Dzyan; U, Tingting; Lay, Shunkay; Jia, Yanbin (2015 yil 7 oktyabr). "Depressiyada oksidlovchi stress belgilarining meta-tahlili". PLOS One. 10 (10): e0138904. Bibcode:2015PLoSO..1038904L. doi:10.1371 / journal.pone.0138904. ISSN 1932-6203. PMC 4596519. PMID 26445247.
- ^ M, Morris G va Berk (2015). "Neyroimmun va neyropsikiyatrik kasalliklarda mitoxondriyal disfunktsiyaga olib boradigan ko'plab yo'llar". BMC tibbiyoti. 13: 68. doi:10.1186 / s12916-015-0310-y. PMC 4382850. PMID 25889215.
- ^ Allen, Josh; Romay-Tallon, Rakel; Braymer, Kayl J.; Caruncho, Hector J.; Kalynchuk, Lisa E. (6 iyun 2018). "Mitoxondriya va kayfiyat: Mitokondriyal disfunktsiya depressiya namoyon bo'lishining asosiy o'yinchisi". Nevrologiya chegaralari. 12: 386. doi:10.3389 / fnins.2018.00386. ISSN 1662-453X. PMC 5997778. PMID 29928190.
- ^ a b v d e Menon, Vinod (2011 yil oktyabr). "Katta miqyosdagi miya tarmoqlari va psixopatologiya: birlashtiruvchi uchlikli tarmoq modeli". Kognitiv fanlarning tendentsiyalari. 15 (10): 483–506. doi:10.1016 / j.tics.2011.08.003. PMID 21908230.
- ^ a b Seli, Vashington; va boshq. (2007 yil fevral). "Zo'rlikni qayta ishlash va ijro etuvchi boshqaruv uchun ajraladigan ichki ulanish tarmoqlari". Neuroscience jurnali. 27 (9): 2349–56. doi:10.1523 / JNEUROSCI.5587-06.2007. PMC 2680293. PMID 17329432.
- ^ Xabas, C; va boshq. (2009 yil 1-iyul). "Ichki aloqa tarmoqlariga serebellar bo'yicha aniq hissa qo'shish". Neuroscience jurnali. 29 (26): 8586–94. doi:10.1523 / JNEUROSCI.1868-09.2009. PMC 2742620. PMID 19571149.
- ^ Petrides, M (2005). "Yanal prefrontal korteks: arxitektura va funktsional tashkilot". Qirollik jamiyatining falsafiy operatsiyalari B. 360 (1456): 781–795. doi:10.1098 / rstb.2005.1631. PMC 1569489. PMID 15937012.
- ^ Vudvord, N.D .; va boshq. (2011). "Shizofreniyada funktsional dam olish holati tarmoqlari turlicha ta'sir qiladi". Shizofreniya tadqiqotlari. 130 (1–3): 86–93. doi:10.1016 / j.schres.2011.03.010. PMC 3139756. PMID 21458238.
- ^ Menon, Vinod; va boshq. (2001). "Shizofreniyada eshitish ishchi xotirasining funktsional neyroanatomiyasi: ijobiy va salbiy alomatlar bilan bog'liqligi". NeuroImage. 13 (3): 433–446. doi:10.1006 / nimg.2000.0699. PMID 11170809.
- ^ Levin, R.L .; va boshq. (2007). "Depressiyadagi kognitiv nuqsonlar va mintaqaviy miya faoliyatining funktsional o'ziga xosligi". Kognitiv terapiya va tadqiqotlar. 31 (2): 211–233. doi:10.1007 / s10608-007-9128-z.
- ^ Qin, P; Northoff, G (2011). "Bizning o'zligimiz o'rta chiziqli mintaqalar va standart rejim tarmog'i bilan qanday bog'liq?". NeuroImage. 57 (3): 1221–1233. doi:10.1016 / j.neuroimage.2011.05.028. PMID 21609772.
- ^ Raichle, M.E .; va boshq. (2001). "Miyaning standart ishlash tartibi". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 98 (2): 676–682. Bibcode:2001 yil PNAS ... 98..676R. doi:10.1073 / pnas.98.2.676. PMC 14647. PMID 11209064.
- ^ Kuni, RE; va boshq. (2010). "Depressiyadagi hayajonlanishning asabiy bog'liqligi". Kognitiv, ta'sirchan va xulq-atvori. 10 (4): 470–478. doi:10.3758 / kabin. 10.4.470. PMC 4476645. PMID 21098808.
- ^ Broyd, S.J .; va boshq. (2009). "Ruhiy kasalliklarda miyaning standart disfunktsiyasi: muntazam ravishda qayta ko'rib chiqish". Neuroscience & Biobehavioral Sharhlar. 33 (3): 279–296. doi:10.1016 / j.neubiorev.2008.09.002. PMID 18824195.
- ^ Xamani, C; va boshq. (2011 yil 15-fevral). "Katta depressiya sharoitida subkalloz singulat girusi". Biologik psixiatriya. 69 (4): 301–8. doi:10.1016 / j.biopsych.2010.09.034. PMID 21145043.
- ^ Feynshteyn, J.S .; va boshq. (2006 yil sentyabr). "Muayyan qarorlar qabul qilishda insulaning oldingi reaktivligi nevrotikizm bilan bog'liq". Ijtimoiy kognitiv va ta'sirchan nevrologiya. 1 (2): 136–142. doi:10.1093 / scan / nsl016. PMC 2555442. PMID 18985124.
- ^ Paulus, M.P; Stein, M.B. (2006). "Xavotirning insular ko'rinishi". Biologik psixiatriya. 60 (4): 383–387. doi:10.1016 / j.biopsych.2006.03.042. PMID 16780813.
- ^ Antoniy, M.M. (2009). Oksfordda bezovtalik va unga bog'liq buzilishlar bo'yicha qo'llanma. Oksford universiteti matbuoti.
Qo'shimcha o'qish
- Szafran, K; Faron-Gorekka, A; Kolasa, M; Kuemider, M; Solich, J; Zuravek, D; Dziedzicka-Wasylewska, M (2013). "G-protein bilan bog'langan retseptorlari (GPCR) heterodimerizatsiyasining nöropsikiyatrik kasalliklarda potentsial roli: depressiyaga e'tibor" (PDF). Farmakol Rep. 65 (6): 1498–505. doi:10.1016 / s1734-1140 (13) 71510-x. PMID 24552997.
- Naumenko, VS; Popova, NK; Lacivita, E; Leopoldo, M; Ponimaskin, EG (2014 yil iyul). "Depressiv kasalliklarda serotonin 5-HT1A va 5-HT7 retseptorlari o'rtasidagi o'zaro bog'liqlik". CNS Neurosci Ther. 20 (7): 582–90. doi:10.1111 / cns.12247. PMC 6493079. PMID 24935787.
