Inson genomi - Human genome
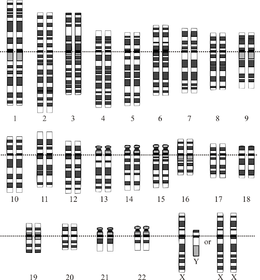 Idealizatsiya qilingan odam diploidining grafik tasviri karyotip, genomning xromosomalarga bo'linishini ko'rsatib beradi. Ushbu rasmda 23-xromosoma juftligining ayol (XX) va erkak (XY) versiyalari ko'rsatilgan. Xromosomalar bir tekisda ko'rsatilgan tsentromeralar. Mitokondriyal DNK ko'rsatilmagan. | |
| NCBI genom identifikatori | 51 |
|---|---|
| Ploidy | diploid |
| Genom hajmi | 3100 Mb / s[1] haploid genomiga (mega-basepairs) to'g'ri keladi Jami 6200 Mbp (diploid). |
| Soni xromosomalar | 23 juft |
The inson genomi to'liq to'plamidir nuklein kislota ketma-ketliklari uchun odamlar sifatida kodlangan DNK 23 ichida xromosoma juftlik hujayra yadrolari va individual tarkibida bo'lgan kichik DNK molekulasida mitoxondriya. Ular odatda yadro genomi sifatida alohida ko'rib chiqiladi va mitoxondriyal genom.[2] Inson genomlar ikkala oqsil kodlovchi DNK genlarini ham o'z ichiga oladi kodlamaydigan DNK. Gaploid tarkibidagi inson genomlari jinsiy hujayralar (the tuxum va sperma jinsiy hujayralar da yaratilgan hujayralar mayoz bosqichi jinsiy ko'payish oldin urug'lantirish yaratadi zigota ) uch milliarddan iborat DNK tayanch juftliklari, esa diploid genomlar ( somatik hujayralar ) DNK tarkibidan ikki baravar ko'p. Odamlar genomlari o'rtasida sezilarli farqlar mavjud (0,1% tartibda tufayli bitta nukleotidli variantlar[3] va ko'rib chiqishda 0,6% indels ),[4] bu odamlar va ularning eng yaqin qarindoshlari o'rtasidagi farqlardan ancha kichikdir bonobos va shimpanze (~1.1% sobit bitta nukleotidli variantlar [5] va indellarni qo'shganda 4%).[6]
Birinchi inson genomlari ketma-ketligi 2001 yil fevral oyida deyarli to'liq qoralama shaklida nashr etilgan Inson genomining loyihasi[7] va Celera korporatsiyasi.[8] Inson genomini loyihalashtirishni ketma-ketlashtirish ishlari yakunlanganligi 2004 yilda genomlar ketma-ketligi nashr etilishi bilan e'lon qilingan bo'lib, ketma-ketlikda atigi 341 bo'shliq qoldirilib, yuqori takrorlanadigan va boshqa DNKni ifodalaydi, o'sha paytda mavjud bo'lgan texnologiya bilan ketma-ketlikni amalga oshirish mumkin emas edi.[9] Inson genomi umurtqali hayvonlar orasida birinchi bo'lib bunday tugallanishga yaqinlashdi va 2018 yilga kelib milliondan ortiq odamlarning diploid genomlari yordamida aniqlandi keyingi avlod ketma-ketligi.[10] Ushbu ma'lumotlar butun dunyo bo'ylab ishlatiladi biotibbiyot fanlari, antropologiya, sud tibbiyoti va boshqa fan sohalari. Bunday genomik tadqiqotlar kasalliklarni tashxislash va davolashda yutuqlarga va ko'plab biologiya sohalarida, shu jumladan yangi tushunchalarga olib keldi inson evolyutsiyasi.
Inson genomining ketma-ketligi (deyarli) to'liq DNK sekvensiyasi bilan aniqlangan bo'lsa-da, u hali to'liq tushunilmagan. Ko'pchilik (ehtimol barchasi ham emas) genlar eksperimental va yuqori samaradorlik kombinatsiyasi bilan aniqlangan bioinformatika yondashuvlar, ammo ularning oqsillari va biologik funktsiyalarini yanada aniqlash uchun hali ko'p ish qilish kerak RNK mahsulotlar. So'nggi natijalar shuni ko'rsatadiki, genom tarkibidagi kodlamaydigan DNKlarning ko'p qismi biokimyoviy faollik, shu jumladan gen ekspressionini tartibga solish, tashkil etish xromosoma me'morchiligi va signallarni boshqarish epigenetik meros.
To'liq genom ketma-ketligini olishdan oldin, inson genlari sonining taxminiy hisob-kitoblari 50,000 dan 140,000 gacha bo'lgan (bu taxminlarga protein bo'lmagan kodlash genlari kiritilganligi to'g'risida vaqti-vaqti bilan noaniqlik bilan).[11] Genomlarning ketma-ketligi sifati va oqsillarni kodlovchi genlarni aniqlash usullari yaxshilanganligi sababli,[9] tan olingan oqsillarni kodlovchi genlar soni 19,000-20,000 gacha tushdi.[12] Biroq, oqsillarni kodlamaydigan, aksincha regulyatorli RNKni ifodalaydigan ketma-ketliklar o'ynaydigan rolni to'liq anglash genlarning umumiy sonini kamida 46,831 ga etkazdi,[13] yana 2300 mikro-RNK geni.[14] 2012 yilga kelib na RNK, na oqsillarni kodlovchi funktsional DNK elementlari qayd etildi.[15] va yaqinda (2018) aholi o'rtasida o'tkazilgan so'rovda inson genomining yana 10% ekvivalenti topildi.[16] Oqsil -kodlash ketma-ketliklari genomning juda oz qismini (taxminan 1,5%) tashkil etadi, qolganlari esa kodlamaydigan RNK genlar, regulyatsion DNK sekanslari, Chiziqlar, Sinuslar, intronlar va hali ketma-ketliklar funktsiya yo'q aniqlandi.[17]
2016 yil iyun oyida olimlar rasmiy ravishda e'lon qilishdi HGP-yozish, inson genomini sintez qilish rejasi.[18][19]
Inson genomlari ketma-ketligining to'liqligi
2001 yilda inson genomini loyihasini yakunlash to'g'risida e'lon qilingan bo'lsa-da,[17] ketma-ketlikda hali ham yuzlab bo'shliqlar mavjud bo'lib, ular qatorining taxminan 5-10% etishmayapti, asosan heteroxromatik mintaqalar va tsentromeralar va telomerlar, lekin ba'zilari ham evromatik mintaqalar.[20] 2015 yilda yana 50 ta ilgari natijalanmagan mintaqalarni qamrab olgan ketma-ketlik aniqlanganda 160 ta evromatik bo'shliq qoldi.[21] Faqatgina 2020 yilda inson xromosomasining birinchi to'liq telomer-telomer ketma-ketligi aniqlandi, ya'ni X xromosoma.[22]
Molekulyar tashkilot va genlarning tarkibi
Odam genomining umumiy uzunligi 3 milliarddan ortiq tayanch juftligini tashkil etadi. Genom 22 juft juft xromosomalarga birlashtirilgan bo'lib, ular termin bilan ataladi autosomalar, ortiqcha 23-juftlik jinsiy xromosomalar (XX) ayolda va (XY) erkakda. Bularning barchasi hujayra yadrosi tarkibidagi katta chiziqli DNK molekulalari. Genom tarkibiga shuningdek kiradi mitoxondrial DNK, har birida mavjud bo'lgan nisbatan kichik dairesel molekula mitoxondriya. A asosidagi ushbu molekulalar va ularning gen tarkibi haqida asosiy ma'lumotlar mos yozuvlar genomi har qanday aniq bir kishining ketma-ketligini anglatmaydigan quyidagi jadvalda keltirilgan. (Ma'lumot manbai: Ensembl genom brauzerining 87-versiyasi[doimiy o'lik havola ], 2016 yilning dekabrida ko'pchilik qadriyatlar uchun; Ensembl genom brauzerining chiqarilishi 68, MiRNA, rRNA, snRNA, snoRNA uchun 2012 yil iyul.)
| Xromosoma | Uzunlik (mm ) | Asosiy juftliklar | O'zgarishlar | Oqsil- kodlash genlar | Pseudo- genlar | Jami uzoq ncRNA | Jami kichik ncRNA | miRNA | rRNK | snRNA | snoRNA | Turli xil ncRNA | Havolalar | Centromere pozitsiya (MB ) | Kümülatif (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | 248,956,422 | 12,151,146 | 2058 | 1220 | 1200 | 496 | 134 | 66 | 221 | 145 | 192 | EBI | 125 | 7.9 |
| 2 | 83 | 242,193,529 | 12,945,965 | 1309 | 1023 | 1037 | 375 | 115 | 40 | 161 | 117 | 176 | EBI | 93.3 | 16.2 |
| 3 | 67 | 198,295,559 | 10,638,715 | 1078 | 763 | 711 | 298 | 99 | 29 | 138 | 87 | 134 | EBI | 91 | 23 |
| 4 | 65 | 190,214,555 | 10,165,685 | 752 | 727 | 657 | 228 | 92 | 24 | 120 | 56 | 104 | EBI | 50.4 | 29.6 |
| 5 | 62 | 181,538,259 | 9,519,995 | 876 | 721 | 844 | 235 | 83 | 25 | 106 | 61 | 119 | EBI | 48.4 | 35.8 |
| 6 | 58 | 170,805,979 | 9,130,476 | 1048 | 801 | 639 | 234 | 81 | 26 | 111 | 73 | 105 | EBI | 61 | 41.6 |
| 7 | 54 | 159,345,973 | 8,613,298 | 989 | 885 | 605 | 208 | 90 | 24 | 90 | 76 | 143 | EBI | 59.9 | 47.1 |
| 8 | 50 | 145,138,636 | 8,221,520 | 677 | 613 | 735 | 214 | 80 | 28 | 86 | 52 | 82 | EBI | 45.6 | 52 |
| 9 | 48 | 138,394,717 | 6,590,811 | 786 | 661 | 491 | 190 | 69 | 19 | 66 | 51 | 96 | EBI | 49 | 56.3 |
| 10 | 46 | 133,797,422 | 7,223,944 | 733 | 568 | 579 | 204 | 64 | 32 | 87 | 56 | 89 | EBI | 40.2 | 60.9 |
| 11 | 46 | 135,086,622 | 7,535,370 | 1298 | 821 | 710 | 233 | 63 | 24 | 74 | 76 | 97 | EBI | 53.7 | 65.4 |
| 12 | 45 | 133,275,309 | 7,228,129 | 1034 | 617 | 848 | 227 | 72 | 27 | 106 | 62 | 115 | EBI | 35.8 | 70 |
| 13 | 39 | 114,364,328 | 5,082,574 | 327 | 372 | 397 | 104 | 42 | 16 | 45 | 34 | 75 | EBI | 17.9 | 73.4 |
| 14 | 36 | 107,043,718 | 4,865,950 | 830 | 523 | 533 | 239 | 92 | 10 | 65 | 97 | 79 | EBI | 17.6 | 76.4 |
| 15 | 35 | 101,991,189 | 4,515,076 | 613 | 510 | 639 | 250 | 78 | 13 | 63 | 136 | 93 | EBI | 19 | 79.3 |
| 16 | 31 | 90,338,345 | 5,101,702 | 873 | 465 | 799 | 187 | 52 | 32 | 53 | 58 | 51 | EBI | 36.6 | 82 |
| 17 | 28 | 83,257,441 | 4,614,972 | 1197 | 531 | 834 | 235 | 61 | 15 | 80 | 71 | 99 | EBI | 24 | 84.8 |
| 18 | 27 | 80,373,285 | 4,035,966 | 270 | 247 | 453 | 109 | 32 | 13 | 51 | 36 | 41 | EBI | 17.2 | 87.4 |
| 19 | 20 | 58,617,616 | 3,858,269 | 1472 | 512 | 628 | 179 | 110 | 13 | 29 | 31 | 61 | EBI | 26.5 | 89.3 |
| 20 | 21 | 64,444,167 | 3,439,621 | 544 | 249 | 384 | 131 | 57 | 15 | 46 | 37 | 68 | EBI | 27.5 | 91.4 |
| 21 | 16 | 46,709,983 | 2,049,697 | 234 | 185 | 305 | 71 | 16 | 5 | 21 | 19 | 24 | EBI | 13.2 | 92.6 |
| 22 | 17 | 50,818,468 | 2,135,311 | 488 | 324 | 357 | 78 | 31 | 5 | 23 | 23 | 62 | EBI | 14.7 | 93.8 |
| X | 53 | 156,040,895 | 5,753,881 | 842 | 874 | 271 | 258 | 128 | 22 | 85 | 64 | 100 | EBI | 60.6 | 99.1 |
| Y | 20 | 57,227,415 | 211,643 | 71 | 388 | 71 | 30 | 15 | 7 | 17 | 3 | 8 | EBI | 10.4 | 100 |
| mtDNA | 0.0054 | 16,569 | 929 | 13 | 0 | 0 | 24 | 0 | 2 | 0 | 0 | 0 | EBI | Yo'q | 100 |
| jami | 3,088,286,401 | 155,630,645 | 20412 | 14600 | 14727 | 5037 | 1756 | 532 | 1944 | 1521 | 2213 |
1-jadval (yuqorida) insonning jismoniy tashkiloti va gen tarkibini umumlashtiradi mos yozuvlar genomi nashr etilganidek, asl tahlilga havolalar bilan Ansambl ma'lumotlar bazasi Evropa bioinformatika instituti (EBI) va Wellcome Trust Sanger instituti. Xromosomalarning uzunligi tayanch juftlari sonini 0,34 nanometrga ko'paytirish orqali aniqlandi, DNK juft spirali. Yangilangan ma'lumotlar asosida inson xromosomalari uzunliklarining yaqinda baholanishi diploid erkak genomi uchun 205,00 sm va ayol uchun 208,23 sm bo'lib, mos ravishda 6,41 va 6,51 pikogramm (pg) vazniga to'g'ri keladi.[23] Oqsillar soni boshlang'ich soniga asoslanadi oldingi mRNK transkriptlar, va mahsulotlarini o'z ichiga olmaydi mRNKgacha muqobil biriktirish, yoki keyin yuzaga keladigan oqsil tuzilishidagi o'zgarishlar tarjima.
Genlarning soni inson genomida to'liq aniq emas, chunki ko'pchilikning vazifasi stenogrammalar noaniqligicha qolmoqda. Bu, ayniqsa, to'g'ri keladi kodlamaydigan RNK (pastga qarang). Oqsillarni kodlovchi genlarning soni yaxshiroq ma'lum, ammo ular funktsional oqsillarni kodlashi mumkin yoki bo'lmasligi mumkin bo'lgan, odatda qisqa muddatlarda kodlangan 1400 ta shubhali genlar qatorida mavjud. ochiq o'qish ramkalari. 2-jadvalda turli xil loyihalar bo'yicha taxminlar keltirilgan va bu nomuvofiqliklar ko'rsatilgan.
| Gencode[25] | Ansambl[26] | Refseq[27] | SHAHMAT[28] | |
|---|---|---|---|---|
| oqsillarni kodlovchi genlar | 19,901 | 20,376 | 20,345 | 21,306 |
| lncRNA genlari | 15,779 | 14,720 | 17,712 | 18,484 |
| antisens RNK | 5501 | 28 | 2694 | |
| turli xil RNK | 2213 | 2222 | 13,899 | 4347 |
| Pseudogenes | 14,723 | 1740 | 15,952 | |
| umumiy transkriptlar | 203,835 | 203,903 | 154,484 | 328,827 |
O'zgarishlar 2016 yil dekabr holatiga ko'ra Ensembl tomonidan tahlil qilingan inson genomlari ketma-ketligida aniqlangan noyob DNK ketma-ketlik farqlari. Belgilangan variatsiyalar soni ortishi kutilmoqda shaxsiy genomlar ketma-ketligi va tahlil qilinganligi. Ushbu jadvalda ko'rsatilgan gen tarkibidan tashqari, inson genomida ko'p miqdordagi ifodalanmagan funktsional ketma-ketliklar aniqlangan (pastga qarang). EBI genom brauzeridagi mos yozuvli xromosoma ketma-ketliklariga oynalarni ochadi.
Kichik kodlamaydigan RNKlar oqsillarni kodlash potentsialiga ega bo'lmagan 200 ga yaqin asoslarning RNKlari. Bunga quyidagilar kiradi: mikroRNKlar yoki miRNA (gen ekspressionining transkripsiyadan keyingi regulyatorlari), kichik yadroli RNKlar, yoki snRNAs (ning RNK tarkibiy qismlari splitseozomalar ) va kichik nukleolyar RNKlar, yoki snoRNA (boshqa RNK molekulalarining kimyoviy modifikatsiyasini boshqarishda ishtirok etadi). Uzoq kodlamaydigan RNKlar oqsillarni kodlash potentsialiga ega bo'lmagan 200 asosdan uzun bo'lgan RNK molekulalari. Bunga quyidagilar kiradi: ribosoma RNKlari, yoki rRNK (ning RNK tarkibiy qismlari ribosomalar ) va boshqa turli xil uzoq RNKlar ishtirok etadi gen ekspressionini tartibga solish, epigenetik DNK nukleotidlarining modifikatsiyalari va histon oqsillar va oqsillarni kodlovchi genlar faoliyatini tartibga solish. Total-small-ncRNA raqamlari va kichik ncNRA'larning ma'lum turlarining raqamlari orasidagi kichik tafovutlar avvalgi qiymatlar Ensembl 87, ikkinchisi Ensembl 68 versiyalari manbalaridan kelib chiqadi.
Axborot tarkibi

The gaploid inson genomi (23 xromosomalar ) taxminan 3 milliard tayanch juftini tashkil etadi va 30000 ga yaqin genni o'z ichiga oladi.[29] Har bir tayanch juftligi 2 bit bilan kodlanishi mumkinligi sababli, bu taxminan 750 ga teng megabayt ma'lumotlar. Shaxsiy somatik (diploid ) hujayra bu miqdorning ikki baravarini, ya'ni taxminan 6 milliard tayanch juftligini o'z ichiga oladi. Erkaklarda ayollarga qaraganda kamroq, chunki Y xromosomasi 57 million asosiy juftni tashkil etadi, X esa taxminan 156 million. Shaxsiy genomlar ketma-ketlikda bir-biridan 1% dan kam farq qilganligi sababli, ma'lum bir odam genomining umumiy ma'lumotnomadagi o'zgarishlari bo'lishi mumkin yo'qotishsiz siqilgan taxminan 4 megabaytgacha.[30]
The entropiya darajasi genomning kodlash va kodlash ketma-ketliklari orasida sezilarli darajada farq qiladi. Kodlash ketma-ketligi uchun har bir tayanch jufti uchun maksimal 2 bitga yaqin (taxminan 45 million tayanch jufti), lekin kodlamaydigan qismlar uchun kamroq. U bitta xromosoma uchun har bir tayanch jufti uchun 1,5 va 1,9 bit oralig'ida, entropiya darajasi har bir juft uchun 0,9 bitdan past bo'lgan Y-xromosomadan tashqari.[31]
Kodlash va DNKni kodlash
Odam genomining tarkibi odatda kodlash va kodlashsiz DNK ketma-ketligiga bo'linadi. DNKni kodlash ko'chirilishi mumkin bo'lgan ketma-ketliklar sifatida aniqlanadi mRNA va tarjima qilingan inson hayoti davomida oqsillarga; bu ketma-ketliklar genomning ozgina qismini egallaydi (<2%). Kodlashsiz DNK oqsillarni kodlash uchun ishlatilmaydigan barcha ketma-ketliklardan (genomning taxminan 98%) iborat.
Ba'zi kodlamaydigan DNKlarda muhim biologik funktsiyalarga ega bo'lgan RNK molekulalari uchun genlar mavjud (kodlashsiz RNK, masalan ribosomal RNK va transfer RNK ). Kodlashsiz DNKning funktsiyasi va evolyutsion kelib chiqishini o'rganish zamonaviy genom tadqiqotlarining muhim maqsadi, shu jumladan KODLASH (DNK elementlari entsiklopediyasi) loyihasi, natijasi molekulyar faollikni ko'rsatadigan turli xil eksperimental vositalardan foydalangan holda, butun inson genomini o'rganishga qaratilgan.
Kodlamaydigan DNK DNKni kodlashdan ancha ustun bo'lganligi sababli, ketma-ket genom tushunchasi DNK-kodlash genining klassik tushunchasiga qaraganda ko'proq yo'naltirilgan analitik tushunchaga aylandi.[32][33]
Kodlash ketma-ketliklari (oqsillarni kodlovchi genlar)
Proteinlarni kodlash ketma-ketliklari inson genomining eng ko'p o'rganilgan va eng yaxshi tushunilgan tarkibiy qismini aks ettiradi. Ushbu ketma-ketliklar oxir-oqibat butun insoniyatning ishlab chiqarishiga olib keladi oqsillar, garchi bir nechta biologik jarayonlar (masalan, DNKni qayta tashkil etish va mRNKgacha muqobil biriktirish ) oqsillarni kodlovchi genlar sonidan ko'ra ko'proq noyob oqsillarni ishlab chiqarishga olib kelishi mumkin. Genomning to'liq modulli oqsillarni kodlash qobiliyati tarkibiga kiradi exome va tomonidan kodlangan DNK sekanslaridan iborat exons bu oqsillarga tarjima qilinishi mumkin. Biologik ahamiyati va genomning 2 foizidan kamrog'ini tashkil etishi sababli ekzomani sekvensiya qilish inson genomi loyihasining birinchi muhim bosqichi edi.
Oqsillarni kodlovchi genlar soni. Kabi ma'lumotlar bazalarida 20000 ga yaqin odam oqsillari izohlangan Uniprot.[35] Tarixiy jihatdan, oqsil genlari bo'yicha hisob-kitoblar juda xilma-xil bo'lib, 1960-yillarning oxirlarida 2.000.000 gacha bo'lgan,[36] Ammo bir necha tadqiqotchilar 1970-yillarning boshlarida taxmin qilingan deb ta'kidladilar mutatsion yuk zararli mutatsiyalardan funktsional lokuslarning umumiy soni uchun taxminan 40 000 yuqori chegara qo'yilgan (bunga protein kodlovchi va funktsional kodlamaydigan genlar kiradi).[37] Odamning oqsillarni kodlovchi genlari soni unchalik murakkab bo'lmagan organizmlarnikidan sezilarli darajada ko'p emas, masalan yumaloq qurt va mevali chivin. Bu farq keng ko'lamli foydalanish natijasida kelib chiqishi mumkin mRNKgacha muqobil biriktirish ekzonlar tanlab qo'shilishi orqali juda ko'p miqdordagi modulli oqsillarni yaratish qobiliyatini ta'minlaydigan odamlarda.
Xromosoma bo'yicha oqsillarni kodlash qobiliyati. Oqsillarni kodlovchi genlar xromosomalar bo'ylab notekis taqsimlanadi, bir necha o'ndan 2000 gacha, ayniqsa yuqori gen zichligi 19, 11 va 1 xromosomalari ichida (1-jadval). Har bir xromosomada turli xil genlarga boy va kambag'al genlar mavjud bo'lib, ular bilan bog'liq bo'lishi mumkin xromosoma polosalari va GK-tarkib.[38] Gen zichligining ushbu tasodifiy naqshlarining ahamiyati yaxshi tushunilmagan.[39]
Oqsillarni kodlovchi genlarning hajmi. Inson genomidagi oqsillarni kodlovchi genlarning hajmi juda katta o'zgaruvchanlikni ko'rsatadi (2-jadval). Oqsillarni kodlovchi genning o'rtacha kattaligi 26,288 bp (o'rtacha = 66,577 bp; jadval 2[40]). Masalan, uchun gen histon H1a (HIST1HIA) nisbatan kichik va sodda, intronlari yo'q va mRNA sekanslarini 781 nt va 215 aminokislota oqsilini (648 nt) kodlaydi. ochiq o'qish doirasi ). Distrofin (DMD) - bu odamning mos yozuvlar genomidagi eng katta oqsil kodlovchi gen bo'lib, umumiy hajmi 2,2 Mb ni tashkil etadi, shu bilan birga Titin (TTN) eng uzun kodlash ketma-ketligiga ega (114,414 bp), eng katta soni exons (363),[41] va eng uzun bitta ekzon (17,106 bp). Butun genom bo'yicha ekzonning o'rtacha kattaligi 122 bp (o'rtacha = 145 bp), ekzonlarning o'rtacha soni 7 (o'rtacha = 8,8), o'rtacha kodlash ketma-ketligi 367 aminokislotani kodlaydi (o'rtacha = 447 aminokislotalar; 21-jadval[17]).
| Oqsil | Xrom | Gen | Uzunlik | Exons | Exon uzunligi | Intron uzunligi | Alt qo'shish |
|---|---|---|---|---|---|---|---|
| Ko'krak bezi saratoniga moyilligi 2-darajali oqsil | 13 | BRCA2 | 83,736 | 27 | 11,386 | 72,350 | ha |
| Kistik fibroz transmembran o'tkazuvchanlik regulyatori | 7 | CFTR | 202,881 | 27 | 4,440 | 198,441 | ha |
| Sitoxrom b | MT | MTCYB | 1,140 | 1 | 1,140 | 0 | yo'q |
| Distrofin | X | DMD | 2,220,381 | 79 | 10,500 | 2,209,881 | ha |
| Gliseraldegid-3-fosfat dehidrogenaza | 12 | GAPDH | 4,444 | 9 | 1,425 | 3,019 | ha |
| Gemoglobin beta birligi | 11 | HBB | 1,605 | 3 | 626 | 979 | yo'q |
| Giston H1A | 6 | HIST1H1A | 781 | 1 | 781 | 0 | yo'q |
| Titin | 2 | TTN | 281,434 | 364 | 104,301 | 177,133 | ha |
Jadval 2. Odamning oqsillarni kodlovchi genlariga misollar. Xrom, xromosoma. Alt qo'shilish, muqobil mRNA qo'shilish. (Ma'lumot manbai: Ensembl genom brauzeri 68-nashr, 2012 yil iyul)
Yaqinda inson genomining yangilangan ma'lumotlarini muntazam ravishda meta-tahlil qilish[40] insonning mos yozuvlar genomidagi eng katta protein-kodlovchi gen ekanligini aniqladi RBFOX1 (RNK bilan bog'lovchi oqsil, tulki-1 gomolog 1), jami 2,47 MB. Butun genom bo'yicha, oqsillarni kodlovchi genlarning tuzilgan to'plamini hisobga olgan holda, ekzonning o'rtacha kattaligi hozirda 133 bp (o'rtacha = 309 bp), ekzonlar o'rtacha soni hozirda 8 (o'rtacha = 11) deb baholanmoqda ) va o'rtacha kodlash ketma-ketligi hozirda 425 aminokislotani (o'rtacha = 553 aminokislotani kodlaydi; 2 va 5-jadvallar[40]).
Kodlamaydigan DNK (ncDNA)
Kodlashsiz DNK genom tarkibidagi barcha DNK ketma-ketliklari deb ta'riflanadi, ular oqsillarni kodlovchi ekzonlar tarkibida mavjud emas va shuning uchun ular hech qachon ifoda etilgan oqsillarning aminokislotalar qatoriga kirmaydi. Ushbu ta'rifga ko'ra, odam genomlarining 98% dan ortig'i ncDNA dan iborat.
Kodlamaydigan DNKning ko'plab sinflari, jumladan kodlanmaydigan RNK (masalan, tRNK va rRNK) uchun genlar, psevdogenlar, intronlar, mRNKning tarjima qilinmagan hududlari, regulyatsion DNK sekanslari, takrorlanadigan DNK sekanslari va mobil genetik elementlar bilan bog'liq ketma-ketliklar aniqlandi.
Genlarga kiritilgan ko'plab ketma-ketliklar, shuningdek, kodlanmagan DNK sifatida aniqlanadi. Bularga kodlamaydigan RNK uchun genlar (masalan, tRNK, rRNK) va oqsil kodlovchi genlarning tarjima qilinmagan tarkibiy qismlari (masalan, intronlar va mRNKning tarjima qilinmagan 5 'va 3' hududlari) kiradi.
Proteinlarni kodlash ketma-ketliklari (xususan, kodlash) exons ) inson genomining 1,5% dan kamini tashkil qiladi.[17] Bundan tashqari, inson genomining taxminan 26% tashkil etadi intronlar.[42] Genlar (ekzonlar va intronlar) va ma'lum tartibga solish sekanslaridan (8-20%) tashqari, inson genomida kodlanmagan DNK mintaqalari mavjud. Hujayra fiziologiyasida rol o'ynaydigan kodlamaydigan DNKning aniq miqdori qizg'in muhokama qilindi. Tomonidan so'nggi tahlillar KODLASH loyiha shuni ko'rsatadiki, butun inson genomining 80% transkripsiyaga uchraydi, tartibga soluvchi oqsillar bilan bog'lanadi yoki boshqa biokimyoviy faollik bilan bog'liq.[15]
Ammo, bu biokimyoviy faollikning barchasi hujayra fiziologiyasiga hissa qo'shadimi yoki uning muhim qismi transkripsiya va biokimyoviy shovqin bo'lib, u organizm tomonidan faol ravishda filtrlanishi kerak.[43] Proteinlarni kodlash ketma-ketliklari, intronlar va tartibga soluvchi mintaqalar bundan mustasno, kodlamaydigan DNKning ko'p qismi quyidagilardan iborat: rol o'ynamaydigan ko'plab DNK sekanslari gen ekspressioni muhim biologik funktsiyalarga ega. Qiyosiy genomika Tadqiqotlar shuni ko'rsatadiki, genomning taxminan 5% yuqori darajadagi kodlanmagan DNK sekanslarini o'z ichiga oladi saqlanib qolgan, ba'zida yuz millionlab yillarni ifodalaydigan vaqt o'lchovlarida bu kodlash mumkin bo'lmagan mintaqalar kuchli ekanligini anglatadi evolyutsion bosim va ijobiy tanlov.[44]
Ushbu ketma-ketliklarning aksariyati xromosomalarning tuzilishini mintaqalarni cheklash orqali tartibga soladi heteroxromatin kabi xromosomalarning tuzilish xususiyatlarini shakllantirish va tartibga solish telomerlar va tsentromeralar. Kodlashtirilmagan boshqa mintaqalar xizmat qiladi DNK replikatsiyasining kelib chiqishi. Nihoyat, bir nechta mintaqalar funktsional kodlanmaydigan RNKga o'tkaziladi, ular oqsillarni kodlovchi genlarning ekspressionini tartibga soladi (masalan,[45] ), mRNA tarjimasi va barqarorligi (qarang miRNA ), xromatin tuzilishi (shu jumladan histon masalan, o'zgartirishlar[46] ), DNK metilatsiyasi (masalan[47] ), DNK rekombinatsiyasi (masalan[48] ) va boshqa kodlamaydigan RNKlarni o'zaro regulyatsiya qilish (masalan, masalan)[49] ). Ehtimol, ko'pgina transkripsiyalangan kodlash bo'lmagan mintaqalar hech qanday rol o'ynamaydi va bu transkripsiya o'ziga xos bo'lmagan mahsulot hisoblanadi. RNK polimeraza faoliyat.[43]
Pseudogenes
Pseudogenes - ko'pincha hosil bo'lgan oqsil kodlovchi genlarning harakatsiz nusxalari genlarning takrorlanishi, inaktivatsiya qiluvchi mutatsiyalar to'planishi natijasida ishlamay qolgan. 1-jadval odam genomidagi pseudogenlar soni 13000 tartibda ekanligini ko'rsatadi,[50] va ba'zi xromosomalarda funktsional protein kodlovchi genlar soni bilan deyarli bir xil. Genlarni ko'paytirish - bu yangi genetik materialni yaratadigan asosiy mexanizm molekulyar evolyutsiya.
Masalan, hidni qabul qiluvchi genlar oilasi - bu inson genomidagi pseudogenlarning eng yaxshi hujjatlashtirilgan namunalaridan biridir. Ushbu oiladagi genlarning 60 foizidan ko'prog'i odamlarda funktsional bo'lmagan psevdogenlardir. Taqqoslash uchun, sichqonchani hidlash retseptorlari genlari oilasidagi genlarning atigi 20 foizi psevdogenlardir. Tadqiqotlar shuni ko'rsatadiki, bu turga xos xususiyatdir, chunki eng yaqin primatlarning barchasi psödogenlarga mutanosib ravishda kamroq bo'ladi. Ushbu genetik kashfiyot boshqa sutemizuvchilarga nisbatan odamlarda kamroq o'tkir hidni tushuntirishga yordam beradi.[51]
Kodlamaydigan RNK (ncRNA) uchun genlar
Kodlamaydigan RNK molekulalari hujayralarda juda muhim rol o'ynaydi, ayniqsa ko'plab reaktsiyalarda oqsil sintezi va RNKni qayta ishlash. Kodlashsiz RNK kiradi tRNK, ribosomal RNK, mikroRNK, snRNA va boshqa kodlamaydigan RNK genlari, shu jumladan taxminan 60,000 uzun bo'lmagan kodlash RNKlari (lncRNAs).[15][52][53][54] Xabar qilingan lncRNA genlari soni ko'payishda davom etsa-da va inson genomidagi aniq son hali aniqlanmagan bo'lsa-da, ularning ko'plari ishlamaydigan deb ta'kidlanmoqda.[55]
Ko'p ncRNAlar genlarni boshqarishda va ekspressionida muhim elementlardir. Kodlashsiz RNK epigenetikaga, transkripsiyaga, RNK qo'shilishiga va translyatsiya mexanizmiga ham hissa qo'shadi. Genetik regulyatsiya va kasallikdagi RNKning roli o'rganilmagan genomik murakkablikning yangi potentsial darajasini taklif etadi.[56]
MRNKning intronlari va tarjima qilinmagan mintaqalari
Diskret genlar tomonidan kodlangan ncRNA molekulalaridan tashqari, oqsillarni kodlovchi genlarning dastlabki transkripsiyalari odatda keng ko'lamli kodlash ketma-ketliklarini o'z ichiga oladi. intronlar, 5'-tarjima qilinmagan mintaqalar (5'-UTR) va 3'-tarjima qilinmagan mintaqalar (3'-UTR). Odam genomining ko'pgina oqsillarni kodlovchi genlarida intron sekanslarining uzunligi ekzon sekanslarining uzunligidan 10-100 baravar ko'pdir (2-jadval).
Regulyatsion DNK sekanslari
Inson genomining xilma-xilligi bor tartibga soluvchi ketma-ketliklar nazorat qilish uchun juda muhimdir gen ekspressioni. Konservativ hisob-kitoblar shuni ko'rsatadiki, bu ketma-ketliklar genomning 8 foizini tashkil qiladi,[57] ammo ekstrapolyatsiyalar KODLASH loyiha 20 ga beradi[58]-40%[59] genomning genlarni tartibga soluvchi ketma-ketligi. Kodlamaydigan DNKlarning ayrim turlari genetik "kalit" lar bo'lib, ular oqsillarni kodlamaydi, lekin genlar qachon va qaerda ifodalanishini tartibga soladi (deyiladi kuchaytirgichlar ).[60]
Tartibga soluvchi ketma-ketliklar 1960 yillarning oxiridan beri ma'lum bo'lgan.[61] Inson genomidagi tartibga soluvchi ketma-ketliklarning birinchi identifikatsiyasi rekombinant DNK texnologiyasiga asoslangan edi.[62] Keyinchalik genomik sekvensiya paydo bo'lishi bilan ushbu ketma-ketliklarni identifikatsiyalash evolyutsion konservatsiya bilan xulosa qilinishi mumkin. Orasidagi evolyutsion tarmoq primatlar va sichqoncha Masalan, 70-90 million yil oldin sodir bo'lgan.[63] Shunday qilib, aniqlaydigan genlar ketma-ketligini kompyuter bilan taqqoslash saqlanmagan kodlash ketma-ketliklari ularning genlarni tartibga solish kabi vazifalardagi ahamiyatiga ishora bo'ladi.[64]
Boshqa genomlar, misol uchun, tabiatni muhofaza qilishga qaratilgan usullarga yordam berish maqsadida ketma-ketlik qilingan puferfish genom.[65] Biroq, tartibga solish ketma-ketliklari yuqori tezlikda evolyutsiya jarayonida yo'qoladi va qayta rivojlanadi.[66][67][68]
2012 yildan boshlab, harakatlar DNK va tartibga soluvchi oqsillarning o'zaro ta'sirini texnikada topishga yo'naltirildi ChIP-seq yoki DNK tomonidan paketlanmagan bo'shliqlar gistonlar (DNase yuqori sezgir saytlar ), ikkalasi ham tekshirilgan hujayra turida faol tartibga soluvchi ketma-ketliklar mavjudligini bildiradi.[57]
DNKning takrorlanadigan ketma-ketliklari
Qayta takrorlanadigan DNK sekanslari inson genomining taxminan 50% ni tashkil qiladi.[69]
Odam genomining taxminan 8% tandem DNK massivlari yoki tandem takrorlashlari, bir nechta qo'shni nusxalarga ega bo'lgan past murakkablikdagi takroriy ketma-ketliklardan iborat (masalan, "CAGCAGCAG ...").[70] Tandem ketma-ketliklari o'zgaruvchan uzunliklarda bo'lishi mumkin, ikkita nukleotiddan o'nlab nukleotidgacha. Ushbu ketma-ketliklar, hatto yaqin qarindosh shaxslar orasida ham juda o'zgaruvchan va shuning uchun ham foydalaniladi genealogik DNK tekshiruvi va sud-tibbiy DNK tahlili.[71]
O'ndan kam nukleotidlardan iborat takroriy ketma-ketliklar (masalan, dinukleotid takrorlanishi (AC))n) mikrosatellitlar ketma-ketligi deb nomlanadi. Mikrosatellit ketma-ketliklari orasida trinukleotid takrorlanishi alohida ahamiyatga ega, chunki ba'zida ular ichida bo'ladi kodlash mintaqalari oqsillar uchun genlar va genetik kasalliklarga olib kelishi mumkin. Masalan, Xantington kasalligi trinukleotid takrorlanishining (CAG) kengayishidan kelib chiqadi.n ichida Huntingtin inson xromosomasidagi gen. Telomerlar (chiziqli xromosomalarning uchlari) ketma-ketlikning mikrosatellit heksanukleotid takrorlanishi bilan tugaydi (TTAGGG)n.
Uzunroq ketma-ketliklarning tandem takrorlanishi (10-60 nukleotid uzunlikdagi takroriy ketma-ketliklar massivi) deyiladi minisellitlar.
Ko'chma genetik elementlar (transpozonlar) va ularning qoldiqlari
Transposable genetik elementlar, O'zlarining nusxalarini xost genomidagi boshqa joylarda takrorlashi va qo'shishi mumkin bo'lgan DNK ketma-ketliklari inson genomida juda ko'p tarkibiy qism hisoblanadi. Eng ko'p tarqalgan transpozon nasli, Alu, taxminan 50,000 faol nusxalari bor,[72] va intragenik va intergenik mintaqalarga kiritilishi mumkin.[73] Boshqa bir nasl-nasab, LINE-1, genom uchun 100 ga yaqin faol nusxaga ega (ularning soni odamlar orasida o'zgarib turadi).[74] Qadimgi transpozonlarning funktsional bo'lmagan qoldiqlari bilan birgalikda ular umumiy DNKning yarmidan ko'pini tashkil qiladi.[75] Ba'zida "sakrash genlari" deb nomlangan transpozonlar inson genomini haykaltaroshlikda katta rol o'ynagan. Ushbu ketma-ketliklarning ba'zilari endogen retroviruslar, Genomga doimiy ravishda singib ketgan va keyingi avlodlarga o'tadigan virusli sekanslarning DNK nusxalari.
Inson genomidagi mobil elementlarni tasniflash mumkin LTR retrotranspozonlari (Umumiy genomning 8,3%), Sinuslar (Umumiy genomning 13,1%), shu jumladan Alu elementlari, Chiziqlar (Umumiy genomning 20,4%), SVA va II sinf DNK transpozonlari (Umumiy genomning 2,9%).
Odamlarning genomik o'zgarishi
Inson ma'lumotnomasi genomi
Bir xil egizaklar bundan mustasno, barcha odamlar genomik DNK ketma-ketliklarida sezilarli o'zgarishlarni ko'rsatmoqdalar. Inson mos yozuvlar genomi (HRG) standart ketma-ketlik ma'lumotnomasi sifatida ishlatiladi.
Insonning murojaat genomiga oid bir nechta muhim fikrlar mavjud:
- HRG - bu gaploid ketma-ketlik. Har bir xromosoma bir martadan ifodalanadi.
- HRG kompozitsion ketma-ketlikdir va har qanday haqiqiy insonga mos kelmaydi.
- HRG vaqti-vaqti bilan xatolarni, noaniqliklarni va noma'lum "bo'shliqlarni" tuzatish uchun yangilanadi.
- HRG hech qanday tarzda "ideal" yoki "mukammal" inson shaxsini anglatmaydi. Bu shunchaki taqqoslash maqsadida ishlatiladigan standartlashtirilgan namoyish yoki model.
The Genom ma'lumotnomasi konsortsiumi HRGni yangilash uchun javobgardir. 38-versiyasi 2013 yil dekabr oyida chiqarilgan.[76]
Insonning genetik o'zgarishini o'lchash
Insonning genetik o'zgarishini tadqiq qilishning aksariyati diqqat markazida bo'lgan bitta nukleotidli polimorfizmlar (SNPs), ular xromosoma bo'ylab alohida asoslarda o'rnini bosuvchi moddalardir. Ko'pgina tahlillar shuni ko'rsatadiki, SNPlar o'rtacha 1000 juft juftlikda, o'rtacha evromatik inson genomi, garchi ular bir xil zichlikda yuzaga kelmasa ham. Shu tariqa ommabop bayonotga amal qilinmoqda: «biz hammamiz, qat'i nazar poyga, genetik jihatdan 99,9% bir xil ",[77] garchi bu ko'pchilik genetiklar tomonidan ma'lum darajada malakaga ega bo'lsa. Masalan, hozirda genomning ancha katta qismi ishtirok etgan deb o'ylashadi nusxa ko'chirish raqamining o'zgarishi.[78] Inson genomidagi SNP turlarini kataloglashtirish bo'yicha keng ko'lamli hamkorlikdagi sa'y-harakatlar Xalqaro HapMap loyihasi.
Kichik turlarning genomik joylari va uzunligi takrorlanadigan ketma-ketliklar ning asosi bo'lgan odamdan odamga juda o'zgaruvchan DNK barmoq izlari va DNKning otalikni tekshirishi texnologiyalar. The heteroxromatik Jami bir necha yuz million tayanch jufti bo'lgan inson genomining qismlari, shuningdek, odam populyatsiyasi ichida juda o'zgaruvchan (ular shunchalik takrorlanadiki va shu qadar uzoqki, ularni hozirgi texnologiya bilan aniq tartiblash mumkin emas). Ushbu mintaqalar oz sonli genlarni o'z ichiga oladi va muhimmi yoki yo'qmi noma'lum fenotipik ta'sir takrorlash yoki heteroxromatinning odatdagi o'zgarishi natijasida hosil bo'ladi.
Eng qo'pol genomik mutatsiyalar jinsiy hujayralar jinsiy hujayralar, ehtimol, ko'rinmas embrionlarni keltirib chiqaradi; ammo, odamlarning bir qator kasalliklari keng ko'lamli genomik anormalliklarga bog'liq. Daun sindromi, Tyorner sindromi, va boshqa bir qator kasalliklar kelib chiqadi mos kelmaydigan butun xromosomalarning Saraton hujayralar tez-tez bor aneuploidiya xromosomalar va xromosoma qo'llari, garchi a sabab va oqibat aneuploidiya va saraton o'rtasidagi munosabatlar o'rnatilmagan.
Insonning genomik o'zgarishini xaritalash
Genom ketma-ketligi genomdagi har bir DNK asosining tartibini sanab o'tgan bo'lsa, genom xaritasi diqqatga sazovor joylarni aniqlaydi. Genom xaritasi genomlar ketma-ketligidan kamroq ma'lumotga ega va genom atrofida harakat qilishda yordam beradi.[79][80]
Variatsiya xaritasiga misol bo'lib, tomonidan ishlab chiqilgan HapMap Xalqaro HapMap loyihasi. HapMap - bu haplotip inson genomining xaritasi, "bu odamning DNK ketma-ketligi o'zgaruvchanligining umumiy naqshlarini tavsiflaydi."[81] U genomdagi bitta DNK harflari yoki bazalarini o'z ichiga olgan kichik o'lchamdagi o'zgarishlarning naqshlarini kataloglaydi.
Tadqiqotchilar jurnalda inson genomidagi keng ko'lamli tarkibiy o'zgarishlarning birinchi ketma-ketlik xaritasini e'lon qilishdi Tabiat 2008 yil may oyida.[82][83] Keng miqyosli strukturaviy o'zgarishlar - bu odamlar orasidagi bir necha mingdan bir necha milliongacha bo'lgan DNK asoslarini o'z ichiga olgan genomdagi farqlar; ba'zilari genomlar ketma-ketligining yutuqlari yoki yo'qotilishlari, boshqalari esa ketma-ketlik cho'zilishlarining qayta tartibga solinishi kabi ko'rinadi. Ushbu o'zgarishlarga quyidagilar kiradi nusxalar sonidagi farqlar jismoniy shaxslar ma'lum bir genga, deletsiyalarga, translokatsiyalarga va inversiyalarga ega.
Odam genomidagi SNP chastotasi
Bitta nukleotidli polimorfizmlar (SNP) inson genomida bir hilda sodir bo'lmaydi. Aslida, unda juda xilma-xillik mavjud SNP har bir genga turli xil selektiv bosimlarni aks ettiruvchi genlar orasidagi chastota, shuningdek genom bo'yicha turli xil mutatsiya va rekombinatsiya stavkalari. Biroq, SNP-lar bo'yicha tadqiqotlar kodlash mintaqalariga to'g'ri kelmaydi, ulardan olingan ma'lumotlar genom bo'ylab SNPlarning umumiy tarqalishini aks ettirishi mumkin emas. Shuning uchun SNP konsortsiumi protokol kodlash mintaqalariga moyilligi bo'lmagan SNPlarni aniqlash uchun ishlab chiqilgan va Konsortsiumning 100000 SNPlari odatda inson xromosomalari bo'yicha ketma-ketlikning xilma-xilligini aks ettiradi. The SNP konsortsiumi 2001 yil birinchi choragining oxiriga kelib genom bo'yicha aniqlangan SNP sonini 300 000 ga etkazishga qaratilgan.[84]
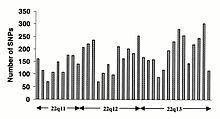
O'zgarishlar kodlamaydigan ketma-ketlik va sinonimik o'zgarishlar kodlash ketma-ketligi odatda sinonimik bo'lmagan o'zgarishlarga qaraganda tez-tez uchraydi, bu aminokislota identifikatorini belgilaydigan pozitsiyalarda xilma-xillikni kamaytiradigan ko'proq selektiv bosimni aks ettiradi. O'tish davri o'zgarishi transversiyalarga qaraganda tez-tez uchraydi, chunki CpG dinukleotidlari mutatsiyaning eng yuqori darajasini, ehtimol deaminatsiyaga bog'liq.
Shaxsiy genomlar
Shaxsiy genomlar ketma-ketligi (deyarli) to'liqdir ketma-ketlik tashkil etuvchi kimyoviy asos juftlarining DNK bitta odamning. Kabi genetik xilma-xillik tufayli tibbiy muolajalar turli odamlarga turlicha ta'sir ko'rsatadi bitta nukleotidli polimorfizmlar (SNP), shaxsiy genomlarni tahlil qilish individual genotiplar asosida shaxsiy tibbiy davolanishga olib kelishi mumkin.[85]
Birinchi shaxsiy genom ketma-ketligi aniqlandi Kreyg Venter 2007 yilda. Shaxsiy genomlar DNK namunalarini taqdim etgan ko'ngillilarning shaxsini himoya qilish bo'yicha "Inson genomlari loyihasi" da tartiblashtirilmagan edi. Ushbu ketma-ketlik turli xil populyatsiyadan bo'lgan bir nechta ko'ngillilarning DNKlaridan olingan.[86] Biroq, Venter boshchiligida Celera Genomics genomni ketma-ketlashtirish bo'yicha harakat, kompozitsion namunani sekvensiyalashdan yakka shaxsdan DNKdan foydalanishga o'tish to'g'risida qaror qabul qilindi, keyinchalik Venterning o'zi ekanligi aniqlandi. Shunday qilib, 2000 yilda chiqarilgan Celera inson genomining ketma-ketligi asosan bitta odamga tegishli edi. Dastlabki kompozitsiyadan olingan ma'lumotlarni keyinchalik almashtirish va ikkala to'plamni ifodalovchi diploid ketma-ketlikni aniqlash xromosomalar Dastlab xabar qilingan gaploid ketma-ketlik o'rniga, birinchi shaxsiy genomni chiqarishga imkon berdi.[87] 2008 yil aprel oyida Jeyms Uotson shuningdek yakunlandi. 2009 yilda Stiven Kveyk o'zining "Heliscope" dizayni bo'yicha sekvensordan olingan o'zining genom ketma-ketligini nashr etdi.[88] Boshchiligidagi Stenford jamoasi Euan Eshli Quake genomida amalga oshirilgan inson genomlarini tibbiy talqini uchun asos yaratdi va birinchi marta butun genomga oid tibbiy qarorlarni qabul qildi.[89] Ushbu jamoa G'arb oilasiga yondashuvni yanada kengaytirdi, Illuminaning shaxsiy genomini ketma-ketlashtirish dasturi doirasida ketma-ket birinchi oila.[90] O'shandan beri yuzlab shaxsiy genomlar ketma-ketligi chiqarildi,[91] shu jumladan Desmond Tutu,[92][93] va a Paleo-Eskimo.[94] 2012 yilda 1092 genom orasida ikkita oilaviy triosning barcha genomlari ketma-ketligi ommaga ma'lum bo'ldi.[3] 2013 yil noyabr oyida ispan oilasi to'rtta shaxsiy ekzome ma'lumotlar to'plamini (genomning taxminan 1%) Creative Commons jamoat mulki litsenziyasi.[95][96] The Shaxsiy genom loyihasi (2005 yilda boshlangan) ikkala genom ketma-ketligini va tegishli tibbiy fenotiplarni ommaga ma'lum qiladiganlar qatoriga kiradi.[97][98]
Ayrim genomlarning ketma-ketligi bundan oldin qadrlanmagan genetik murakkablik darajasini yanada oshkor qildi. Shaxsiy genomika nafaqat SNPlarga, balki tarkibiy o'zgarishlarga ham bog'liq bo'lgan inson genomidagi xilma-xillikning muhim darajasini aniqlashga yordam berdi. Biroq, bunday bilimlarni kasalliklarni davolashda va tibbiyot sohasida qo'llash faqat uning boshlanishida.[99] Exome ketma-ketligi genetik kasallikni aniqlashda yordam beradigan vosita sifatida tobora ommalashib bormoqda, chunki ekzome genomik ketma-ketlikning atigi 1 foizini tashkil qiladi, ammo kasalliklarga sezilarli darajada ta'sir etuvchi mutatsiyalarning taxminan 85 foizini tashkil qiladi.[100]
Inson nokautlari
Odamlarda, genlarni nokaut qilish tabiiy ravishda yuzaga keladi heterozigot yoki bir jinsli funktsiyani yo'qotish genlarni nokaut qilish. Ushbu nokautlarni ko'pincha, ayniqsa, ichkarida farqlash qiyin heterojen genetik kelib chiqishi. They are also difficult to find as they occur in low frequencies.
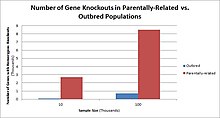
Populations with high rates of qarindoshlik, such as countries with high rates of first-cousin marriages, display the highest frequencies of homozygous gene knockouts. Such populations include Pakistan, Iceland, and Amish populations. These populations with a high level of parental-relatedness have been subjects of human knock out research which has helped to determine the function of specific genes in humans. By distinguishing specific knockouts, researchers are able to use phenotypic analyses of these individuals to help characterize the gene that has been knocked out.
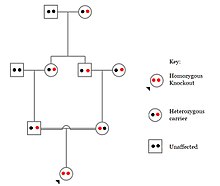
Knockouts in specific genes can cause genetic diseases, potentially have beneficial effects, or even result in no phenotypic effect at all. However, determining a knockout's phenotypic effect and in humans can be challenging. Challenges to characterizing and clinically interpreting knockouts include difficulty calling of DNA variants, determining disruption of protein function (annotation), and considering the amount of influence mosaicism has on the phenotype.[101]
One major study that investigated human knockouts is the Pakistan Risk of Myocardial Infarction study. It was found that individuals possessing a heterozygous loss-of-function gene knockout for the APOC3 gene had lower triglycerides in the blood after consuming a high fat meal as compared to individuals without the mutation. However, individuals possessing homozygous loss-of-function gene knockouts of the APOC3 gene displayed the lowest level of triglycerides in the blood after the fat load test, as they produce no functional APOC3 protein.[102]
Human genetic disorders
Most aspects of human biology involve both genetic (inherited) and non-genetic (environmental) factors. Some inherited variation influences aspects of our biology that are not medical in nature (height, eye color, ability to taste or smell certain compounds, etc.). Moreover, some genetic disorders only cause disease in combination with the appropriate environmental factors (such as diet). With these caveats, genetic disorders may be described as clinically defined diseases caused by genomic DNA sequence variation. In the most straightforward cases, the disorder can be associated with variation in a single gene. Masalan, kistik fibroz is caused by mutations in the CFTR gene and is the most common recessive disorder in caucasian populations with over 1,300 different mutations known.[103]
Disease-causing mutations in specific genes are usually severe in terms of gene function and are fortunately rare, thus genetic disorders are similarly individually rare. However, since there are many genes that can vary to cause genetic disorders, in aggregate they constitute a significant component of known medical conditions, especially in pediatric medicine. Molecularly characterized genetic disorders are those for which the underlying causal gene has been identified. Currently there are approximately 2,200 such disorders annotated in the OMIM ma'lumotlar bazasi.[103]
Studies of genetic disorders are often performed by means of family-based studies. In some instances, population based approaches are employed, particularly in the case of so-called founder populations such as those in Finland, French-Canada, Utah, Sardinia, etc. Diagnosis and treatment of genetic disorders are usually performed by a genetik -physician trained in clinical/medical genetics. Natijalari Inson genomining loyihasi are likely to provide increased availability of genetik test for gene-related disorders, and eventually improved treatment. Parents can be screened for hereditary conditions and counselled on the consequences, the probability of inheritance, and how to avoid or ameliorate it in their offspring.
There are many different kinds of DNA sequence variation, ranging from complete extra or missing chromosomes down to single nucleotide changes. It is generally presumed that much naturally occurring genetic variation in human populations is phenotypically neutral, i.e., has little or no detectable effect on the physiology of the individual (although there may be fractional differences in fitness defined over evolutionary time frames). Genetic disorders can be caused by any or all known types of sequence variation. To molecularly characterize a new genetic disorder, it is necessary to establish a causal link between a particular genomic sequence variant and the clinical disease under investigation. Such studies constitute the realm of human molecular genetics.
With the advent of the Human Genome and Xalqaro HapMap loyihasi, it has become feasible to explore subtle genetic influences on many common disease conditions such as diabetes, asthma, migraine, schizophrenia, etc. Although some causal links have been made between genomic sequence variants in particular genes and some of these diseases, often with much publicity in the general media, these are usually not considered to be genetic disorders o'z-o'zidan as their causes are complex, involving many different genetic and environmental factors. Thus there may be disagreement in particular cases whether a specific medical condition should be termed a genetic disorder.
Additional genetic disorders of mention are Kallman syndrome va Pfeiffer sindromi (gene FGFR1), Fuchs corneal dystrophy (gene TCF4), Hirschsprung kasalligi (genes RET and FECH), Bardet-Biedl syndrome 1 (genes CCDC28B and BBS1), Bardet-Biedl syndrome 10 (gene BBS10), and facioscapulohumeral muscular dystrophy type 2 (genes D4Z4 and SMCHD1).[104]
Genome sequencing is now able to narrow the genome down to specific locations to more accurately find mutations that will result in a genetic disorder. Copy number variants (CNVs) and single nucleotide variants (SNVs) are also able to be detected at the same time as genome sequencing with newer sequencing procedures available, called Next Generation Sequencing (NGS). This only analyzes a small portion of the genome, around 1-2%. The results of this sequencing can be used for clinical diagnosis of a genetic condition, including Usher sindromi, retinal disease, hearing impairments, diabetes, epilepsy, Leigh disease, hereditary cancers, neuromuscular diseases, primary immunodeficiencies, og'ir birlashgan immunitet tanqisligi (SCID), and diseases of the mitochondria.[105] NGS can also be used to identify carriers of diseases before conception. The diseases that can be detected in this sequencing include Tay-Sachs disease, Bloom syndrome, Gaucher disease, Canavan disease, familial dysautonomia, cystic fibrosis, o'murtqa mushak atrofiyasi va fragile-X syndrome. The Next Genome Sequencing can be narrowed down to specifically look for diseases more prevalent in certain ethnic populations.[106]
The categorized table below provides the prevalence as well as the genes or chromosomes associated with some human genetic disorders.
| Buzuqlik | Tarqalishi | Chromosome or gene involved |
|---|---|---|
| Chromosomal conditions | ||
| Daun sindromi | 1:600 | 21-xromosoma |
| Klinefelter sindromi | 1:500–1000 males | Additional X chromosome |
| Tyorner sindromi | 1:2000 females | Loss of X chromosome |
| O'roqsimon hujayra anemiyasi | 1 in 50 births in parts of Africa; rarer elsewhere | β-globin (on chromosome 11) |
| Bloom syndrome | 1:48000 Ashkenazi Jews | BLM |
| Saraton | ||
| Ko'krak /Tuxumdon saratoni (susceptibility) | ~5% of cases of these cancer types | BRCA1, BRCA2 |
| FAP (hereditary nonpolyposis coli) | 1:3500 | APC |
| Lynch sindromi | 5–10% of all cases of bowel cancer | MLH1, MSH2, MSH6, PMS2 |
| Fankoni anemiyasi | 1:130000 births | MUXLIS |
| Neurological conditions | ||
| Xantington kasalligi | 1:20000 | Huntingtin |
| Altsgeymer kasalligi ‐ early onset | 1:2500 | PS1, PS2, APP |
| Tay-Sachs | 1:3600 births in Ashkenazi Jews | HEXA gene (on chromosome 15) |
| Canavan disease | 2.5% Eastern European Jewish ancestry | ASPA gene (on chromosome 17) |
| Familial dysautonomia | 600 known cases worldwide since discovery | IKBKAP gene (on chromosome 9) |
| Mo'rt X sindromi | 1.4:10000 in males, 0.9:10000 in females | FMR1 gene (on X chromosome) |
| Mukolipidoz IV turi | 1:90 to 1:100 in Ashkenazi Jews | MCOLN1 |
| Boshqa shartlar | ||
| Kistik fibroz | 1:2500 | CFTR |
| Duxenne mushak distrofiyasi | 1:3500 boys | Distrofin |
| Becker muscular dystrophy | 1.5-6:100000 males | DMD |
| Beta talassemiya | 1:100000 | HBB |
| Tug'ma buyrak usti hiperplaziyasi | 1:280 in Native Americans and Yupik Eskimos 1:15000 in American Caucasians | CYP21A2 |
| Glikogenni saqlash kasalligi I turi | 1:100000 births in America | G6PC |
| Maple siropi siydik kasalligi | 1:180000 in the U.S. 1:176 in Mennonite/Amish communities 1:250000 in Austria | BCKDHA BCKDHB DBT DLD |
| Niemann–Pick disease, SMPD1-associated | 1,200 cases worldwide | SMPD1 |
| Usher sindromi | 1:23000 in the U.S. 1:28000 in Norway 1:12500 in Germany | CDH23 MYO7A PCDH15 USH1C USH1G USH2A GPR98 DFNB31 CLRN1 |
Evolyutsiya
Comparative genomics studies of mammalian genomes suggest that approximately 5% of the human genome has been conserved by evolution since the divergence of extant lineages approximately 200 million years ago, containing the vast majority of genes.[107][108] The published shimpanze genome differs from that of the human genome by 1.23% in direct sequence comparisons.[109] Around 20% of this figure is accounted for by variation within each species, leaving only ~1.06% consistent sequence divergence between humans and chimps at shared genes.[110] This nucleotide by nucleotide difference is dwarfed, however, by the portion of each genome that is not shared, including around 6% of functional genes that are unique to either humans or chimps.[111]
In other words, the considerable observable differences between humans and chimps may be due as much or more to genome level variation in the number, function and expression of genes rather than DNA sequence changes in shared genes. Indeed, even within humans, there has been found to be a previously unappreciated amount of copy number variation (CNV) which can make up as much as 5 – 15% of the human genome. In other words, between humans, there could be +/- 500,000,000 base pairs of DNA, some being active genes, others inactivated, or active at different levels. The full significance of this finding remains to be seen. On average, a typical human protein-coding gene differs from its chimpanzee ortholog by only two aminokislota substitutions; nearly one third of human genes have exactly the same protein translation as their chimpanzee orthologs. A major difference between the two genomes is human xromosoma 2, which is equivalent to a fusion product of chimpanzee chromosomes 12 and 13.[112] (later renamed to chromosomes 2A and 2B, respectively).
Humans have undergone an extraordinary loss of olfactory receptor genes during our recent evolution, which explains our relatively crude sense of hid compared to most other mammals. Evolutionary evidence suggests that the emergence of rangni ko'rish in humans and several other primat species has diminished the need for the sense of smell.[113]
In September 2016, scientists reported that, based on human DNA genetic studies, all non-Africans in the world today can be traced to a single population bu exited Africa between 50,000 and 80,000 years ago.[114]
Mitoxondrial DNK
Inson mitoxondrial DNK is of tremendous interest to geneticists, since it undoubtedly plays a role in mitochondrial disease. It also sheds light on human evolution; for example, analysis of variation in the human mitochondrial genome has led to the postulation of a recent common ancestor for all humans on the maternal line of descent (see Mitoxondrial Momo Havo ).
Due to the lack of a system for checking for copying errors,[iqtibos kerak ] mitochondrial DNA (mtDNA) has a more rapid rate of variation than nuclear DNA. This 20-fold[tekshirish kerak ] higher mutation rate allows mtDNA to be used for more accurate tracing of maternal ancestry.[iqtibos kerak ] Studies of mtDNA in populations have allowed ancient migration paths to be traced, such as the migration of Mahalliy amerikaliklar dan Sibir[iqtibos kerak ] yoki Polineziyaliklar from southeastern Osiyo[iqtibos kerak ]. It has also been used to show that there is no trace of Neandertal DNA in the European gene mixture inherited through purely maternal lineage.[115] Due to the restrictive all or none manner of mtDNA inheritance, this result (no trace of Neanderthal mtDNA) would be likely unless there were a large percentage of Neanderthal ancestry, or there was strong positive selection for that mtDNA (for example, going back 5 generations, only 1 of your 32 ancestors contributed to your mtDNA, so if one of these 32 was pure Neanderthal you would expect that ~3% of your autosomal DNA would be of Neanderthal origin, yet you would have a ~97% chance to have no trace of Neanderthal mtDNA).[iqtibos kerak ]
Epigenome
Epigenetics describes a variety of features of the human genome that transcend its primary DNA sequence, such as kromatin packaging, histon modifications and DNK metilatsiyasi, and which are important in regulating gene expression, genome replication and other cellular processes. Epigenetic markers strengthen and weaken transcription of certain genes but do not affect the actual sequence of DNA nucleotides. DNA methylation is a major form of epigenetic control over gene expression and one of the most highly studied topics in epigenetics. During development, the human DNA methylation profile experiences dramatic changes. In early germ line cells, the genome has very low methylation levels. These low levels generally describe active genes. As development progresses, parental imprinting tags lead to increased methylation activity.[116][117]
Epigenetic patterns can be identified between tissues within an individual as well as between individuals themselves. Identical genes that have differences only in their epigenetic state are called epialleles. Epialleles can be placed into three categories: those directly determined by an individual's genotype, those influenced by genotype, and those entirely independent of genotype. The epigenome is also influenced significantly by environmental factors. Diet, toxins, and hormones impact the epigenetic state. Studies in dietary manipulation have demonstrated that methyl-deficient diets are associated with hypomethylation of the epigenome. Such studies establish epigenetics as an important interface between the environment and the genome.[118]
Shuningdek qarang
Adabiyotlar
- ^ "GRCh38.p13". ncbi. Genom ma'lumotnomasi konsortsiumi. Olingan 8 iyun 2020.
- ^ Brown TA (2002). The Human Genome (2-nashr). Oxford: Wiley-Liss.
- ^ a b Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA (November 2012). "An integrated map of genetic variation from 1,092 human genomes". Tabiat. 491 (7422): 56–65. Bibcode:2012Natur.491...56T. doi:10.1038/nature11632. PMC 3498066. PMID 23128226.
- ^ Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. (Oktyabr 2015). "A global reference for human genetic variation". Tabiat. 526 (7571): 68–74. Bibcode:2015Natur.526...68T. doi:10.1038/nature15393. PMC 4750478. PMID 26432245.
- ^ Chimpanzee Sequencing; Analysis Consortium (2005). "Initial sequence of the chimpanzee genome and comparison with the human genome" (PDF). Tabiat. 437 (7055): 69–87. Bibcode:2005Natur.437...69.. doi:10.1038/nature04072. PMID 16136131. S2CID 2638825.
- ^ Varki A, Altheide TK (December 2005). "Comparing the human and chimpanzee genomes: searching for needles in a haystack". Genom tadqiqotlari. 15 (12): 1746–58. doi:10.1101/gr.3737405. PMID 16339373.
- ^ International Human Genome Sequencing Consortium Publishes Sequence and Analysis of the Human Genome
- ^ Pennisi E (February 2001). "The human genome". Ilm-fan. 291 (5507): 1177–80. doi:10.1126/science.291.5507.1177. PMID 11233420. S2CID 38355565.
- ^ a b International Human Genome Sequencing Consortium (October 2004). "Finishing the euchromatic sequence of the human genome". Tabiat. 431 (7011): 931–45. Bibcode:2004Natur.431..931H. doi:10.1038/nature03001. PMID 15496913.
- ^ Molteni M (19 November 2018). "Now You Can Sequence Your Whole Genome For Just $200". Simli.
- ^ Wade N (23 September 1999). "Number of Human Genes Is Put at 140,000, a Significant Gain". The New York Times.
- ^ Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML (November 2014). "Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes". Inson molekulyar genetikasi. 23 (22): 5866–78. doi:10.1093/hmg/ddu309. PMC 4204768. PMID 24939910.
- ^ Saey TH (17 September 2018). "A recount of human genes ups the number to at least 46,831". Fan yangiliklari.
- ^ Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, et al. (Aprel 2019). "An estimate of the total number of true human miRNAs". Nuklein kislotalarni tadqiq qilish. 47 (7): 3353–3364. doi:10.1093/nar/gkz097. PMC 6468295. PMID 30820533.
- ^ a b v Pennisi E (September 2012). "Genomics. ENCODE project writes eulogy for junk DNA". Ilm-fan. 337 (6099): 1159–1161. doi:10.1126/science.337.6099.1159. PMID 22955811.
- ^ Zhang S (28 November 2018). "300 Million Letters of DNA Are Missing From the Human Genome". Atlantika.
- ^ a b v d International Human Genome Sequencing Consortium (February 2001). "Inson genomini dastlabki ketma-ketligi va tahlili". Tabiat. 409 (6822): 860–921. Bibcode:2001 yil Natur.409..860L. doi:10.1038/35057062. PMID 11237011.CS1 maint: mualliflar parametridan foydalanadi (havola)
- ^ Pollack A (2 June 2016). "Scientists Announce HGP-Write, Project to Synthesize the Human Genome". Nyu-York Tayms. Olingan 2 iyun 2016.
- ^ Boeke JD, Church G, Hessel A, Kelley NJ, Arkin A, Cai Y, et al. (2016 yil iyul). "The Genome Project-Write". Ilm-fan. 353 (6295): 126–7. Bibcode:2016Sci...353..126B. doi:10.1126/science.aaf6850. PMID 27256881. S2CID 206649424.
- ^ Zhang, Sarah (28 November 2018). "300 Million Letters of DNA Are Missing From the Human Genome". Atlantika. Olingan 16 avgust 2019.
- ^ Chaisson MJ, Huddleston J, Dennis MY, Sudmant PH, Malig M, Hormozdiari F, Antonacci F, Surti U, Sandstrom R, Boitano M, Landolin JM, Stamatoyannopoulos JA, Hunkapiller MW, Korlach J, Eichler EE (January 2015). "Resolving the complexity of the human genome using single-molecule sequencing". Tabiat. 517 (7536): 608–11. Bibcode:2015Natur.517..608C. doi:10.1038/nature13907. PMC 4317254. PMID 25383537.
- ^ Miga, Karen H.; Koren, Sergey; Rhie, Arang; Vollger, Mitchell R.; Gershman, Ariel; Bzikadze, Andrey; Brooks, Shelise; Howe, Edmund; Porubsky, David; Logsdon, Glennis A.; Schneider, Valerie A. (September 2020). "Telomere-to-telomere assembly of a complete human X chromosome". Tabiat. 585 (7823): 79–84. doi:10.1038/s41586-020-2547-7. ISSN 1476-4687. PMC 7484160. PMID 32663838.
- ^ Piovesan A, Pelleri MC, Antonaros F, Strippoli P, Caracausi M, Vitale L (February 2019). "On the length, weight and GC content of the human genome". BMC tadqiqotlari bo'yicha eslatmalar. 12 (1): 106. doi:10.1186/s13104-019-4137-z. PMC 6391780. PMID 30813969.
- ^ Salzberg SL (August 2018). "Open questions: How many genes do we have?". BMC biologiyasi. 16 (1): 94. doi:10.1186/s12915-018-0564-x. PMC 6100717. PMID 30124169.
- ^ "Gencode statistics, version 28". Arxivlandi asl nusxasi 2018 yil 2 martda. Olingan 12 iyul 2018.
- ^ "Ensemble statistics for version 92.38, corresponding to Gencode v28". Olingan 12 iyul 2018.
- ^ "NCBI Homo sapiens Annotation Release 108". NIH. 2016 yil.
- ^ "CHESS statistics, version 2.0". Hisoblash biologiyasi markazi. Jons Xopkins universiteti.
- ^ "Human Genome Project Completion: Frequently Asked Questions". National Human Genome Research Institute (NHGRI). Olingan 2 fevral 2019.
- ^ Christley S, Lu Y, Li C, Xie X (January 2009). "Human genomes as email attachments". Bioinformatika. 25 (2): 274–5. doi:10.1093/bioinformatics/btn582. PMID 18996942.
- ^ Liu Z (2008). "Sequence space coverage, entropy of genomes and the potential to detect non-human DNA in human samples". BMC Genomics. 9: 509. doi:10.1186/1471-2164-9-509. PMC 2628393. PMID 18973670., Anjir. 6, using the Lempel-Ziv estimators of entropy rate.
- ^ Waters K (7 March 2007). "Molecular Genetics". Stenford falsafa entsiklopediyasi. Olingan 18 iyul 2013.
- ^ Gannett L (26 October 2008). "The Human Genome Project". Stenford falsafa entsiklopediyasi. Olingan 18 iyul 2013.
- ^ PANTHER Pie Chart at the PANTHER Classification System homepage. Retrieved 25 May 2011
- ^ List of human proteins in the Uniprot Human reference proteome; accessed 28 January 2015
- ^ Kauffman SA (March 1969). "Metabolic stability and epigenesis in randomly constructed genetic nets". Nazariy biologiya jurnali. 22 (3): 437–67. doi:10.1016/0022-5193(69)90015-0. PMID 5803332.
- ^ Ohno S (1972). "An argument for the genetic simplicity of man and other mammals". Inson evolyutsiyasi jurnali. 1 (6): 651–662. doi:10.1016/0047-2484(72)90011-5.
- ^ Sémon M, Mouchiroud D, Duret L (February 2005). "Relationship between gene expression and GC-content in mammals: statistical significance and biological relevance". Inson molekulyar genetikasi. 14 (3): 421–7. doi:10.1093/hmg/ddi038. PMID 15590696.
- ^ M. Huang, H. Zhu, B. Shen, G. Gao, "A non-random gait through the human genome", 3rd International Conference on Bioinformatics and Biomedical Engineering (UCBBE, 2009), 1–3
- ^ a b v Piovesan A, Caracausi M, Antonaros F, Pelleri MC, Vitale L (2016). "GeneBase 1.1: a tool to summarize data from NCBI gene datasets and its application to an update of human gene statistics". Database: The Journal of Biological Databases and Curation. 2016: baw153. doi:10.1093/database/baw153. PMC 5199132. PMID 28025344.
- ^ Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S (2001). "The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system". Sirkulyatsiya tadqiqotlari. 89 (11): 1065–72. doi:10.1161/hh2301.100981. PMID 11717165.
- ^ Gregory TR (September 2005). "Synergy between sequence and size in large-scale genomics". Genetika haqidagi sharhlar. 6 (9): 699–708. doi:10.1038/nrg1674. PMID 16151375. S2CID 24237594.
- ^ a b Palazzo AF, Akef A (June 2012). "Nuclear export as a key arbiter of "mRNA identity" in eukaryotes". Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 1819 (6): 566–77. doi:10.1016/j.bbagrm.2011.12.012. PMID 22248619.
- ^ Ludwig MZ (December 2002). "Functional evolution of noncoding DNA". Genetika va rivojlanishning dolzarb fikri. 12 (6): 634–9. doi:10.1016/S0959-437X(02)00355-6. PMID 12433575.
- ^ Martens JA, Laprade L, Winston F (June 2004). "Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene". Tabiat. 429 (6991): 571–4. Bibcode:2004Natur.429..571M. doi:10.1038/nature02538. PMID 15175754. S2CID 809550.
- ^ Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY (Avgust 2010). "Long noncoding RNA as modular scaffold of histone modification complexes". Ilm-fan. 329 (5992): 689–93. Bibcode:2010Sci...329..689T. doi:10.1126/science.1192002. PMC 2967777. PMID 20616235.
- ^ Bartolomei MS, Zemel S, Tilghman SM (May 1991). "Parental imprinting of the mouse H19 gene". Tabiat. 351 (6322): 153–5. Bibcode:1991Natur.351..153B. doi:10.1038/351153a0. PMID 1709450. S2CID 4364975.
- ^ Kobayashi T, Ganley AR (September 2005). "Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats". Ilm-fan. 309 (5740): 1581–4. Bibcode:2005Sci...309.1581K. doi:10.1126/science.1116102. PMID 16141077. S2CID 21547462.
- ^ Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP (August 2011). "A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?". Hujayra. 146 (3): 353–8. doi:10.1016/j.cell.2011.07.014. PMC 3235919. PMID 21802130.
- ^ Pei B, Sisu C, Frankish A, Howald C, Habegger L, Mu XJ, Harte R, Balasubramanian S, Tanzer A, Diekhans M, Reymond A, Hubbard TJ, Harrow J, Gerstein MB (2012). "The GENCODE pseudogene resource". Genom biologiyasi. 13 (9): R51. doi:10.1186/gb-2012-13-9-r51. PMC 3491395. PMID 22951037.
- ^ Gilad Y, Man O, Pääbo S, Lancet D (March 2003). "Human specific loss of olfactory receptor genes". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 100 (6): 3324–7. Bibcode:2003PNAS..100.3324G. doi:10.1073/pnas.0535697100. PMC 152291. PMID 12612342.
- ^ Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM (March 2015). "The landscape of long noncoding RNAs in the human transcriptome". Tabiat genetikasi. 47 (3): 199–208. doi:10.1038/ng.3192. PMC 4417758. PMID 25599403.
- ^ Eddy SR (December 2001). "Non-coding RNA genes and the modern RNA world". Genetika haqidagi sharhlar. 2 (12): 919–29. doi:10.1038/35103511. PMID 11733745. S2CID 18347629.
- ^ Managadze D, Lobkovsky AE, Wolf YI, Shabalina SA, Rogozin IB, Koonin EV (2013). "The vast, conserved mammalian lincRNome". PLOS hisoblash biologiyasi. 9 (2): e1002917. Bibcode:2013PLSCB...9E2917M. doi:10.1371/journal.pcbi.1002917. PMC 3585383. PMID 23468607.
- ^ Palazzo AF, Lee ES (2015). "Non-coding RNA: what is functional and what is junk?". Genetika chegaralari. 6: 2. doi:10.3389/fgene.2015.00002. PMC 4306305. PMID 25674102.
- ^ Mattick JS, Makunin IV (April 2006). "Non-coding RNA". Inson molekulyar genetikasi. 15 Spec No 1: R17–29. doi:10.1093/hmg/ddl046. PMID 16651366.
- ^ a b Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M (September 2012). "An integrated encyclopedia of DNA elements in the human genome". Tabiat. 489 (7414): 57–74. Bibcode:2012Natur.489...57T. doi:10.1038/nature11247. PMC 3439153. PMID 22955616.
- ^ Birney E (5 September 2012). "ENCODE: My own thoughts". Ewan's Blog: Bioinformatician at large.
- ^ Stamatoyannopoulos JA (September 2012). "What does our genome encode?". Genom tadqiqotlari. 22 (9): 1602–11. doi:10.1101/gr.146506.112. PMC 3431477. PMID 22955972.
- ^ Carroll SB, Gompel N, Prudhomme B (May 2008). "Regulating Evolution". Ilmiy Amerika. 298 (5): 60–67. Bibcode:2008SciAm.298e..60C. doi:10.1038/scientificamerican0508-60. PMID 18444326.
- ^ Miller JH, Ippen K, Scaife JG, Beckwith JR (1968). "The promoter-operator region of the lac operon of Escherichia coli". J. Mol. Biol. 38 (3): 413–20. doi:10.1016/0022-2836(68)90395-1. PMID 4887877.
- ^ Wright S, Rosenthal A, Flavell R, Grosveld F (1984). "DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells". Hujayra. 38 (1): 265–73. doi:10.1016/0092-8674(84)90548-8. PMID 6088069. S2CID 34587386.
- ^ Nei M, Xu P, Glazko G (February 2001). "Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 98 (5): 2497–502. Bibcode:2001PNAS...98.2497N. doi:10.1073/pnas.051611498. PMC 30166. PMID 11226267.
- ^ Loots GG, Locksley RM, Blankespoor CM, Wang ZE, Miller W, Rubin EM, Frazer KA (April 2000). "Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons". Ilm-fan. 288 (5463): 136–40. Bibcode:2000Sci...288..136L. doi:10.1126/science.288.5463.136. PMID 10753117.Xulosa
- ^ Meunier M. "Genoscope and Whitehead announce a high sequence coverage of the Tetraodon nigroviridis genome". Genoscope. Arxivlandi asl nusxasi 2006 yil 16 oktyabrda. Olingan 12 sentyabr 2006.
- ^ Romero IG, Ruvinsky I, Gilad Y (July 2012). "Comparative studies of gene expression and the evolution of gene regulation". Genetika haqidagi sharhlar. 13 (7): 505–16. doi:10.1038/nrg3229. PMC 4034676. PMID 22705669.
- ^ Schmidt D, Wilson MD, Ballester B, Schwalie PC, Brown GD, Marshall A, Kutter C, Watt S, Martinez-Jimenez CP, Mackay S, Talianidis I, Flicek P, Odom DT (May 2010). "Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding". Ilm-fan. 328 (5981): 1036–40. Bibcode:2010Sci...328.1036S. doi:10.1126/science.1186176. PMC 3008766. PMID 20378774.
- ^ Wilson MD, Barbosa-Morais NL, Schmidt D, Conboy CM, Vanes L, Tybulewicz VL, Fisher EM, Tavaré S, Odom DT (October 2008). "Species-specific transcription in mice carrying human chromosome 21". Ilm-fan. 322 (5900): 434–8. Bibcode:2008Sci...322..434W. doi:10.1126/science.1160930. PMC 3717767. PMID 18787134.
- ^ Treangen TJ, Salzberg SL (January 2012). "Repetitive DNA and next-generation sequencing: computational challenges and solutions". Genetika haqidagi sharhlar. 13 (1): 36–46. doi:10.1038/nrg3117. PMC 3324860. PMID 22124482.
- ^ Duitama J, Zablotskaya A, Gemayel R, Jansen A, Belet S, Vermeesch JR, Verstrepen KJ, Froyen G (May 2014). "Large-scale analysis of tandem repeat variability in the human genome". Nuklein kislotalarni tadqiq qilish. 42 (9): 5728–41. doi:10.1093/nar/gku212. PMC 4027155. PMID 24682812.
- ^ Pierce BA (2012). Genetics : a conceptual approach (4-nashr). Nyu-York: W.H. Freeman. pp. 538–540. ISBN 978-1-4292-3250-0.
- ^ Bennett EA, Keller H, Mills RE, Schmidt S, Moran JV, Weichenrieder O, Devine SE (December 2008). "Active Alu retrotransposons in the human genome". Genom tadqiqotlari. 18 (12): 1875–83. doi:10.1101/gr.081737.108. PMC 2593586. PMID 18836035.
- ^ Liang KH, Yeh CT (2013). "A gene expression restriction network mediated by sense and antisense Alu sequences located on protein-coding messenger RNAs". BMC Genomics. 14: 325. doi:10.1186/1471-2164-14-325. PMC 3655826. PMID 23663499.
- ^ Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH (April 2003). "Hot L1s account for the bulk of retrotransposition in the human population". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 100 (9): 5280–5. Bibcode:2003PNAS..100.5280B. doi:10.1073/pnas.0831042100. PMC 154336. PMID 12682288.
- ^ Barton NH, Briggs DE, Eisen JA, Goldstein DB, Patel NH (2007). Evolyutsiya. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-684-9.
- ^ NCBI. "GRCh38 – hg38 – Genome – Assembly – NCBI". ncbi.nlm.nih.gov. Olingan 15 mart 2019.
- ^ "from Bill Clinton's 2000 State of the Union address". Arxivlandi asl nusxasi 2017 yil 21 fevralda. Olingan 14 iyun 2007.
- ^ Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. (2006 yil noyabr). "Global variation in copy number in the human genome". Tabiat. 444 (7118): 444–54. Bibcode:2006Natur.444..444R. doi:10.1038/nature05329. PMC 2669898. PMID 17122850.
- ^ "What's a Genome?". Genomenewsnetwork.org. 2003 yil 15-yanvar. Olingan 31 may 2009.
- ^ NCBI_user_services (29 March 2004). "Mapping Factsheet". Ncbi.nlm.nih.gov. Arxivlandi asl nusxasi 2010 yil 19-iyulda. Olingan 31 may 2009.
- ^ "Loyiha to'g'risida". HapMap. Olingan 31 may 2009.
- ^ "2008 Release: Researchers Produce First Sequence Map of Large-Scale Structural Variation in the Human Genome". genome.gov. Olingan 31 may 2009.
- ^ Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, Graves T, et al. (2008 yil may). "Mapping and sequencing of structural variation from eight human genomes". Tabiat. 453 (7191): 56–64. Bibcode:2008Natur.453...56K. doi:10.1038/nature06862. PMC 2424287. PMID 18451855.
- ^ Gray IC, Campbell DA, Spurr NK (2000). "Single nucleotide polymorphisms as tools in human genetics". Inson molekulyar genetikasi. 9 (16): 2403–2408. doi:10.1093/hmg/9.16.2403. PMID 11005795.
- ^ Lai E (June 2001). "Application of SNP technologies in medicine: lessons learned and future challenges". Genom tadqiqotlari. 11 (6): 927–9. doi:10.1101/gr.192301. PMID 11381021.
- ^ "Human Genome Project Completion: Frequently Asked Questions". genome.gov. Olingan 31 may 2009.
- ^ Singer E (4 September 2007). "Craig Venter's Genome". MIT Technology Review. Olingan 25 may 2010.
- ^ Pushkarev, Dmitry; Neff, Norma F; Quake, Stephen R (September 2009). "Single-molecule sequencing of an individual human genome". Tabiat biotexnologiyasi. 27 (9): 847–850. doi:10.1038/nbt.1561.
- ^ Ashley, Euan A; Butte, Atul J; Wheeler, Matthew T; Chen, Rong; Klein, Teri E; Dewey, Frederick E; Dudley, Joel T; Ormond, Kelly E; Pavlovic, Aleksandra; Morgan, Alexander A; Pushkarev, Dmitry; Neff, Norma F; Hudgins, Louanne; Gong, Li; Hodges, Laura M; Berlin, Dorit S; Thorn, Caroline F; Sangkuhl, Katrin; Hebert, Joan M; Woon, Mark; Sagreiya, Hersh; Whaley, Ryan; Knowles, Joshua W; Chou, Michael F; Thakuria, Joseph V; Rosenbaum, Abraham M; Zaranek, Alexander Wait; Church, George M; Greely, Henry T; Quake, Stephen R; Altman, Russ B (May 2010). "Clinical assessment incorporating a personal genome". Lanset. 375 (9725): 1525–1535. doi:10.1016/S0140-6736(10)60452-7.
- ^ Dewey, Frederick E.; Chen, Rong; Cordero, Sergio P.; Ormond, Kelly E.; Caleshu, Colleen; Karczewski, Konrad J.; Whirl-Carrillo, Michelle; Wheeler, Matthew T.; Dudley, Joel T.; Byrnes, Jake K.; Cornejo, Omar E.; Knowles, Joshua W.; Woon, Mark; Sangkuhl, Katrin; Gong, Li; Thorn, Caroline F.; Hebert, Joan M.; Capriotti, Emidio; David, Sean P.; Pavlovic, Aleksandra; West, Anne; Thakuria, Joseph V.; Ball, Madeleine P.; Zaranek, Alexander W.; Rehm, Heidi L.; Church, George M.; West, John S.; Bustamante, Carlos D.; Snayder, Maykl; Altman, Russ B.; Klein, Teri E.; Butte, Atul J.; Ashley, Euan A. (15 September 2011). "Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence". PLoS Genetika. 7 (9): e1002280. doi:10.1371/journal.pgen.1002280.
- ^ "Complete Genomics Adds 29 High-Coverage, Complete Human Genome Sequencing Datasets to Its Public Genomic Repository".
- ^ Sample I (17 February 2010). "Desmond Tutu's genome sequenced as part of genetic diversity study". The Guardian.
- ^ Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, Kasson LR, et al. (2010). "Complete Khoisan and Bantu genomes from southern Africa". Tabiat. 463 (7283): 943–7. Bibcode:2010Natur.463..943S. doi:10.1038/nature08795. PMC 3890430. PMID 20164927.
- ^ Rasmussen M, Li Y, Lindgreen S, Pedersen JS, Albrechtsen A, Moltke I, et al. (2010 yil fevral). "Ancient human genome sequence of an extinct Palaeo-Eskimo". Tabiat. 463 (7282): 757–62. Bibcode:2010Natur.463..757R. doi:10.1038/nature08835. PMC 3951495. PMID 20148029.
- ^ Corpas M, Cariaso M, Coletta A, Weiss D, Harrison AP, Moran F, Yang H (12 November 2013). "A Complete Public Domain Family Genomics Dataset". bioRxiv 10.1101/000216.
- ^ Corpas M (2013 yil iyun). "Crowdsourcing the corpasome". Source Code for Biology and Medicine. 8 (1): 13. doi:10.1186/1751-0473-8-13. PMC 3706263. PMID 23799911.
- ^ Mao Q, Ciotlos S, Zhang RY, Ball MP, Chin R, Carnevali P, et al. (Oktyabr 2016). "The whole genome sequences and experimentally phased haplotypes of over 100 personal genomes". GigaScience. 5 (1): 42. doi:10.1186/s13742-016-0148-z. PMC 5057367. PMID 27724973.
- ^ Cai B, Li B, Kiga N, Thusberg J, Bergquist T, Chen YC, et al. (September 2017). "Matching phenotypes to whole genomes: Lessons learned from four iterations of the personal genome project community challenges". Inson mutatsiyasi. 38 (9): 1266–1276. doi:10.1002/humu.23265. PMC 5645203. PMID 28544481.
- ^ Gonzaga-Jauregui C, Lupski JR, Gibbs RA (2012). "Human genome sequencing in health and disease". Annual Review of Medicine. 63: 35–61. doi:10.1146/annurev-med-051010-162644. PMC 3656720. PMID 22248320.
- ^ Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloğlu A, Ozen S, Sanjad S, Nelson-Williams C, Farhi A, Mane S, Lifton RP (November 2009). "Genetic diagnosis by whole exome capture and massively parallel DNA sequencing". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 106 (45): 19096–101. Bibcode:2009PNAS..10619096C. doi:10.1073/pnas.0910672106. PMC 2768590. PMID 19861545.
- ^ a b Narasimhan VM, Xue Y, Tyler-Smith C (April 2016). "Human Knockout Carriers: Dead, Diseased, Healthy, or Improved?". Molekulyar tibbiyot tendentsiyalari. 22 (4): 341–351. doi:10.1016/j.molmed.2016.02.006. PMC 4826344. PMID 26988438.
- ^ Saleheen D, Natarajan P, Armean IM, Zhao W, Rasheed A, Khetarpal SA, et al. (2017 yil aprel). "Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity". Tabiat. 544 (7649): 235–239. Bibcode:2017Natur.544..235S. doi:10.1038/nature22034. PMC 5600291. PMID 28406212.
- ^ a b Hamosh A, Scott AF, Amberger J, Bocchini C, Valle D, McKusick VA (January 2002). "Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders". Nuklein kislotalarni tadqiq qilish. 30 (1): 52–5. doi:10.1093/nar/30.1.52. PMC 99152. PMID 11752252.
- ^ Katsanis N (November 2016). "The continuum of causality in human genetic disorders". Genom biologiyasi. 17 (1): 233. doi:10.1186/s13059-016-1107-9. PMC 5114767. PMID 27855690.
- ^ Wong, Lee-Jun C. (2017), Wong, Lee-Jun C. (ed.), "Overview of the Clinical Utility of Next Generation Sequencing in Molecular Diagnoses of Human Genetic Disorders", Next Generation Sequencing Based Clinical Molecular Diagnosis of Human Genetic Disorders, Springer International Publishing, pp. 1–11, doi:10.1007/978-3-319-56418-0_1, ISBN 978-3-319-56418-0
- ^ Fedick A, Zhang J (2017). Wong LC (ed.). Next Generation of Carrier Screening. Next Generation Sequencing Based Clinical Molecular Diagnosis of Human Genetic Disorders. Springer xalqaro nashriyoti. pp. 339–354. doi:10.1007/978-3-319-56418-0_16. ISBN 978-3-319-56418-0.
- ^ Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, et al. (2002 yil dekabr). "Initial sequencing and comparative analysis of the mouse genome". Tabiat. 420 (6915): 520–62. Bibcode:2002Natur.420..520W. doi:10.1038/nature01262. PMID 12466850.
the proportion of small (50–100 bp) segments in the mammalian genome that is under (purifying) selection can be estimated to be about 5%. This proportion is much higher than can be explained by protein-coding sequences alone, implying that the genome contains many additional features (such as untranslated regions, regulatory elements, non-protein-coding genes, and chromosomal structural elements) under selection for biological function.
- ^ Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, et al. (2007 yil iyun). "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Tabiat. 447 (7146): 799–816. Bibcode:2007Natur.447..799B. doi:10.1038/nature05874. PMC 2212820. PMID 17571346.
- ^ The Chimpanzee Sequencing; Analysis Consortium (September 2005). "Initial sequence of the chimpanzee genome and comparison with the human genome". Tabiat. 437 (7055): 69–87. Bibcode:2005Natur.437...69.. doi:10.1038/nature04072. PMID 16136131.
We calculate the genome-wide nucleotide divergence between human and chimpanzee to be 1.23%, confirming recent results from more limited studies.
- ^ The Chimpanzee Sequencing; Analysis Consortium (September 2005). "Shimpanze genomining dastlabki ketma-ketligi va odam genomiga taqqoslash". Tabiat. 437 (7055): 69–87. Bibcode:2005 yil 537 ... 69.. doi:10.1038 / nature04072. PMID 16136131.
polimorfizm kuzatilgan divergentsiya darajasining 14-22% ni tashkil qiladi va shu bilan sobit divergentsiya ~ 1,06% yoki undan kam
- ^ Demuth JP, De Bie T, Stajich JE, Cristianini N, Hahn MW (2006). "Sutemizuvchilar genlari oilalari evolyutsiyasi". PLOS ONE. 1 (1): e85. Bibcode:2006PLoSO ... 1 ... 85D. doi:10.1371 / journal.pone.0000085. PMC 1762380. PMID 17183716.
Bizning natijalarimiz shuni anglatadiki, odamlar va shimpanzelar o'zlarining gen komplemanida kamida 6% (22000 genning 1418 tasi) bilan farq qiladi, bu esa ortologik nukleotidlar ketma-ketligi o'rtasidagi tez-tez keltirilgan 1,5% farqdan qat'iy farq qiladi.
- ^ Shimpanzening ketma-ketligi; Tahlil konsortsiumi (2005 yil sentyabr). "Shimpanze genomining dastlabki ketma-ketligi va odam genomiga taqqoslash". Tabiat. 437 (7055): 69–87. Bibcode:2005 yil 537 ... 69.. doi:10.1038 / nature04072. PMID 16136131.
Inson xromosomasi 2 chimpanze nasabida alohida qolgan ikkita ajdodlar xromosomalarining birlashishi natijasida paydo bo'ldi.
Olson MV, Varki A (2003 yil yanvar). "Shimpanze genomining ketma-ketligi: inson evolyutsiyasi va kasalliklari to'g'risida tushunchalar". Genetika haqidagi sharhlar. 4 (1): 20–8. doi:10.1038 / nrg981. PMID 12509750. S2CID 205486561.Hozir shimpanze genomining katta hajmdagi sekvensiyasi yaqinlashmoqda.
- ^ Gilad Y, Viebe V, Prjevorski M, Lanset D, Pääbo S (yanvar 2004). "Xushbo'y retseptorlari genlarini yo'qotish primatlarda to'liq trikromatik ko'rish qobiliyatiga ega bo'lish bilan mos keladi". PLOS biologiyasi. 2 (1): E5. doi:10.1371 / journal.pbio.0020005. PMC 314465. PMID 14737185.
Bizning topilmalarimiz hidlash repertuarining yomonlashuvi primatlarda to'liq trikromatik rangli ko'rinishga ega bo'lish bilan bir vaqtda sodir bo'lganligini ko'rsatadi.
- ^ Zimmer C (2016 yil 21 sentyabr). "Biz bu erga qanday etib keldik: DNK Afrikadan yagona ko'chishga ishora qilmoqda". Nyu-York Tayms. Olingan 22 sentyabr 2016.
- ^ Sykes B (2003 yil 9 oktyabr). "Mitokondriyal DNK va insoniyat tarixi". Inson genomi. Arxivlandi asl nusxasi 2015 yil 7 sentyabrda. Olingan 19 sentyabr 2006.
- ^ Misteli T (2007 yil fevral). "Ketma-ketlikdan tashqari: genom funktsiyasini uyali tashkil etish". Hujayra. 128 (4): 787–800. doi:10.1016 / j.cell.2007.01.028. PMID 17320514. S2CID 9064584.
- ^ Bernshteyn BE, Meissner A, Lander ES (fevral 2007). "Sutemizuvchilar epigenomasi". Hujayra. 128 (4): 669–81. doi:10.1016 / j.cell.2007.01.033. PMID 17320505. S2CID 2722988.
- ^ Scheen AJ, Junien C (2012 yil may-iyun). "[Epigenetika, atrof-muhit va genlar o'rtasidagi aloqa: murakkab kasalliklarda roli]". Medicale de Liège revue. 67 (5–6): 250–7. PMID 22891475.
