Alvocidib - Alvocidib
 | |
 | |
| Ismlar | |
|---|---|
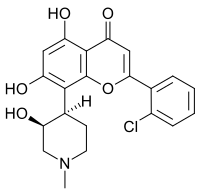
| IUPAC nomi 2- (2-xlorofenil) -5,7-dihidroksi-8 - [(3S, 4R) -3-gidroksi-1-metil-4-piperidinil] -4-xromenon | |
| Boshqa ismlar Flavopiridol, HMR 1275, L-868275 | |
| Identifikatorlar | |
3D model (JSmol ) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | Flavopiridol |
PubChem CID | |
| UNII | |
CompTox boshqaruv paneli (EPA) | |
| |
| |
| Xususiyatlari | |
| C21H20ClNO5 | |
| Molyar massa | 401.8402 |
Boshqacha ko'rsatilmagan hollar bundan mustasno, ulardagi materiallar uchun ma'lumotlar berilgan standart holat (25 ° C [77 ° F], 100 kPa da). | |
| Infobox ma'lumotnomalari | |
Alvocidib (KARVONSAROY; shuningdek, nomi bilan tanilgan Flavopiridol) a flavonoid alkaloid CDK9 kinaz inhibitori davolash uchun klinik rivojlanish ostida o'tkir miyeloid leykemiya, tomonidan Tolero Pharmaceuticals, Inc. Davolash uchun ham o'rganilgan artrit[1] va aterosklerotik blyashka shakllanish[2] Flavopiridolning maqsadi ijobiy transkripsiyaning uzayish omilidir P-TEFb.[3][4] Flavopiridol bilan hujayralarni davolash P-TEFb inhibisyoniga va yo'qotilishiga olib keladi mRNA ishlab chiqarish.[5][6]
Murakkab tabiiy mahsulotning sintetik analogidir rohitukin dastlab olingan Amoora rohituka [sin. Aphanamixis polystachya ] va undan keyin Dysoxylum binectariferum.[7][8]
Yetim dori
Tolero farmatsevtika Inc FDA tomonidan berilganligini e'lon qildi yetim dori Alvocidib, uning siklinga bog'liq kinaz kichik molekula inhibitori, bemorlarni davolash uchun belgilanishi o'tkir miyeloid leykemiya.[9]
Adabiyotlar
- ^ Sekine C, Sugihara T, Miyake S, Hirai H, Yoshida M, Miyasaka N, Kohsaka H (2008). "Romatoid artritning hayvonot modellarini kichik molekulali siklinga bog'liq kinaz inhibitörleri bilan muvaffaqiyatli davolash". J. Immunol. 180 (3): 1954–61. doi:10.4049 / jimmunol.180.3.1954. PMID 18209094.
- ^ Ruef J, Meshel AS, Xu Z, Horaist C, Ballinger CA, Tompson LJ, Subbarao VD, Dyumont JA, Patterson S (1999). "Flavopiridol in vitro va neointimal shakllanishda silliq mushak hujayralari ko'payishini inhibe qiladi. Sichqonchada karotid shikastlangandan so'ng in vivo jonli". Sirkulyatsiya. 100 (6): 659–65. doi:10.1161 / 01.cir.100.6.659. PMID 10441105.
- ^ Chao SH, Fujinaga K, Marion JE, Taube R, Sausvill EA, Senderowicz AM, Peterlin BM, Narx DH (2000). "Flavopiridol P-TEFb ni inhibe qiladi va OIV-1 replikatsiyasini bloklaydi". J. Biol. Kimyoviy. 275 (37): 28345–8. doi:10.1074 / jbc.C000446200. PMID 10906320.
- ^ Chao SH, Narx DH (2001). "Flavopiridol P-TEFb ni inaktiv qiladi va in Vivo jonli ravishda RNK polimeraza II transkripsiyasini bloklaydi". J. Biol. Kimyoviy. 276 (34): 31793–9. doi:10.1074 / jbc.M102306200. PMID 11431468.
- ^ Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Xu X, Gnatt A, Young RA, Narx DH (2012). "Gdown1 ning inson genlariga asoslangan RNK polimeraza II bilan funktsional assotsiatsiyasi". Mol. Hujayra. 45 (1): 38–50. doi:10.1016 / j.molcel.2011.10.022. PMC 3259526. PMID 22244331.
- ^ Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA (2010). "c-Myc transkripsiyaviy pauza chiqarilishini tartibga soladi". Hujayra. 141 (3): 432–45. doi:10.1016 / j.cell.2010.03.030. PMC 2864022. PMID 20434984.
- ^ Harmon, AD; Vayss, U; Silverton, QK (1979). "Rohitukinning tuzilishi, Amora rohitukaning asosiy alkaloidi (syn.Aphanamixis polystachya) (Meliaceae)". Tetraedr Lett. 20 (1): 721–724. doi:10.1016 / S0040-4039 (01) 93556-7.
- ^ Lakdawala, AD; Shirole, MV; Mandrekar, SS; Dohadwalla, AN (1988). "Rohitukinning immunofarmakologik salohiyati: Dysoxylum binectariferum o'simlikidan ajratilgan yangi birikma". Asia Pac J Pharmcol. 3 (1): 91–98.
- ^ http://www.healio.com/hematology-oncology/hematologic-malignancies/news/online/%7B74c6a69e-4529-400d-98e9-d5ee6c602122%7D/fda-grants-orphan-drug-status-to-alvocidib-for -aml
| Bu antineoplastik yoki immunomodulyatsion dori maqola a naycha. Siz Vikipediyaga yordam berishingiz mumkin uni kengaytirish. |
