Ozonning yemirilishi - Ozone depletion
| Tashqi audio | |
|---|---|
 | |
Ozonning yemirilishi o'tgan asrning 70-yillari oxiridan e'tiboran kuzatilgan ikkita hodisadan iborat: ularning umumiy miqdoridagi to'rt foizga doimiy pasayish ozon yilda Yerning atmosfera ( ozon qatlami ) va bahor faslining pasayishi ancha katta stratosfera Yerning qutbli mintaqalari atrofidagi ozon.[1] Oxirgi hodisa ozon teshigi. Shuningdek, bahorgi qutblar mavjud troposfera ozon qatlamining buzilishi hodisalari bu stratosfera hodisalariga qo'shimcha ravishda.
Ozon parchalanishi va ozon teshigining asosiy sababi ishlab chiqarilgan kimyoviy moddalardir, ayniqsa ishlab chiqarilgan halokarbon sovutgichlar, erituvchilar, yonilg'i quyish vositalari va ko'pik- shamollatuvchi vositalar (xloroflorokarbonatlar (CFCs), HCFCs, halonlar ) deb nomlanadi ozonni emiruvchi moddalar (ODS). Ushbu birikmalar stratosferaga turbulent aralashtirish sirtdan chiqqandan so'ng, molekulalar joylashgandan ancha tezroq aralashtiriladi.[2] Stratosferaga kirib, ular orqali halogen atomlarini chiqaradi fotodissotsiatsiya, qaysi kataliz qiling ozonning parchalanishi (O3) kislorodga (O2).[3] Ozon qatlamining ikkala turi ham halokarbonat emissiyasi oshishi bilan ortib borishi kuzatildi.
Ozonning emirilishi va ozon teshigi dunyo bo'ylab saraton xavfining oshishi va boshqa salbiy ta'sirlardan xavotirga sabab bo'ldi. Ozon qatlami eng zararli to'lqin uzunliklarining oldini oladi ultrabinafsha (UV) nurlari Yer atmosferasi. Ushbu to'lqin uzunliklari sabab bo'ladi teri saratoni, quyosh yonishi, doimiy ko'rlik va katarakt, ular ozonning ingichkalashi, shuningdek o'simliklar va hayvonlarga zarar etkazishi natijasida keskin ko'payishi taxmin qilingan. Ushbu tashvishlar qabul qilinishiga olib keldi Monreal protokoli 1987 yilda CFC, halon va ozonni emiruvchi boshqa kimyoviy moddalarni ishlab chiqarishni taqiqlaydi.
Taqiq 1989 yilda kuchga kirdi. Ozon darajasi 90-yillarning o'rtalarida barqarorlashdi va 2000-yillarda tiklana boshladi, chunki reaktiv oqim janubiy yarimsharda janubiy qutb tomon to'xtadi va hatto orqaga qaytishi mumkin.[4] Qayta tiklash kelgusi asrda davom etishi va ozon teshigi 2075 yilga kelib 1980 yilgacha bo'lgan darajaga yetishi kutilmoqda.[5] 2019 yilda, NASA ozon teshigi birinchi marta 1982 yilda kashf etilganidan beri eng kichik bo'lganligi haqida xabar berdi.[6][7][8]
Monreal protokoli bugungi kungacha eng muvaffaqiyatli xalqaro ekologik kelishuv hisoblanadi.[9][10]
Ozon siklining umumiy ko'rinishi
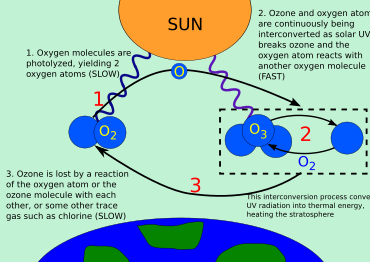
Uch shakl (yoki.) allotroplar ) ning kislorod bilan bog'liq ozon-kislorod aylanishi: kislorod atomlari (O yoki atomik kislorod), kislorodli gaz (O
2 yoki diatomik kislorod) va ozon gazi (O
3 yoki uch atomli kislorod). Ozon kislorod molekulalari ultrabinafsha fotonlarni yutgandan so'ng fotodissotsialashganda stratosferada hosil bo'ladi. Bu bitta o'zgartiradi O
2 ikki atomik kislorodga aylanadi radikallar. Keyinchalik atomik kislorod radikallari alohida bilan birikadi O
2 ikkitasini yaratish uchun molekulalar O
3 molekulalar. Ushbu ozon molekulalari ultrabinafsha nurlarini yutadi, so'ngra ozon molekulasiga bo'linadi O
2 va kislorod atomi. Keyin kislorod atomi ozonni qayta tiklash uchun kislorod molekulasi bilan birlashadi. Bu kislorod atomi ozon molekulasi bilan qayta birikib, ikkitasini hosil qilganda to'xtaydigan davom etadigan jarayondir O
2 molekulalar.
O + O
3 → 2 O
2
Stratosferadagi ozonning umumiy miqdori fotokimyoviy ishlab chiqarish va rekombinatsiya o'rtasidagi muvozanat bilan belgilanadi.
Ozon bir qator tomonidan yo'q qilinishi mumkin erkin radikal katalizatorlar; eng muhimi gidroksil radikal (OH ·), azot oksidi radikal (NO ·), xlor radikal (Cl ·) va brom radikal (Br ·). Nuqta - bu har bir turning juftlashtirilmagan elektronga ega ekanligini va shu bilan nihoyatda reaktiv ekanligini ko'rsatuvchi belgi. Bularning barchasi tabiiy va sun'iy manbalarga ega; hozirgi paytda stratosferadagi OH · va NO · ning katta qismi tabiiy ravishda uchraydi, ammo inson faoliyati xlor va brom miqdorini keskin oshirdi[11]. Ushbu elementlar barqaror organik birikmalarda, ayniqsa xloroflorokarbonatlar, ularning reaktivligi pastligi tufayli troposferada vayron bo'lmasdan stratosferaga borishi mumkin. Stratosferada bo'lganidan so'ng, Cl va Br atomlari ultrabinafsha nurlar ta'sirida ota-ona birikmalaridan ajralib chiqadi, masalan.
CFCl
3 + elektromagnit nurlanish → Cl · + ·CFCl
2
Ozon katalizator yordamida ancha barqaror kislorod shaklini osonlikcha kamaytiradigan yuqori reaktiv molekuladir. Cl va Br atomlari ozon molekulalarini turli xil moddalar yordamida yo'q qiladi katalitik tsikllar. Bunday tsiklning eng oddiy misolida,[12] xlor atomi ozon molekulasi bilan reaksiyaga kirishadi (O
3), xlor oksidi (ClO) hosil qilish uchun kislorod atomini olib, kislorod molekulasini qoldirib (O
2). ClO ozonning ikkinchi molekulasi bilan reaksiyaga kirishib, xlor atomini chiqarib, ikki molekula kislorod hosil qilishi mumkin. Ushbu gaz fazali reaktsiyalar uchun kimyoviy stenografiya:
- Cl · + O
3 → ClO + O
2
Xlor atomi ozon molekulasidan kislorod atomini chiqarib ClO molekulasini hosil qiladi - ClO + O
3 → Cl · + 2 O
2
Ushbu ClO boshqa ozon molekulasidan kislorod atomini ham chiqarib yuborishi mumkin; xlor bu ikki bosqichli tsiklni takrorlash uchun bepul
Umumiy ta'sir ozon miqdorining pasayishiga olib keladi, ammo bu jarayonlarning tezligi ta'sirida kamayishi mumkin nol davrlar. Bundan tashqari, quyi stratosferada ozonni yo'q qilishga olib keladigan yanada murakkab mexanizmlar topildi.
Bitta xlor atomi ozonni (shu bilan katalizatorni) ikki yilgacha doimiy ravishda yo'q qilishi mumkin edi (troposferaga qaytish uchun vaqt o'lchovi), agar ularni ushbu tsikldan olib tashlaydigan reaktsiyalar bo'lmasa, masalan, suv omborlari turlarini hosil qilish. vodorod xlorid (HCl) va xlor nitrat (KLONO
2). Brom ozonni atomga qarab yo'q qilishda xlordan ham samaraliroq, ammo hozirgi vaqtda atmosferada brom juda kam. Xlor ham, brom ham ozonning emirilishiga katta hissa qo'shadi. Laboratoriya tadqiqotlari shuni ko'rsatdiki, ftor va yod atomlari o'xshash katalitik tsikllarda ishtirok etadi. Shu bilan birga, ftor atomlari suv va metan bilan tezda reaksiyaga kirishib, qattiq bog'langan hosil bo'ladi HF yod o'z ichiga olgan organik molekulalar atmosferaning pastki qismida shunchalik tez reaksiyaga kirishadiki, ular stratosferaga sezilarli darajada etib bormaydilar.
Bitta xlor atomi katalitik tsikldan chiqarilishidan oldin o'rtacha 100000 ozon molekulalari bilan reaksiyaga kirisha oladi. Xloroflorokarbonlar (KFKlar) va gidroxloroflorokarbonatlar (HCFC) tomonidan atmosferaga har yili chiqarilgan xlor miqdori va bu atrof-muhit uchun KFC va XSKK xavfliligini namoyish etadi.[13][14]
Ozon qatlamining parchalanishiga oid kuzatishlar

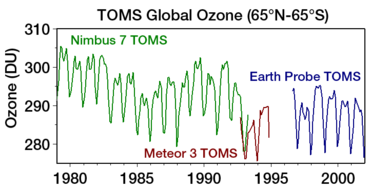
Ozon teshigi odatda jami kamayish bilan o'lchanadi ustunli ozon Yer yuzidagi bir nuqtadan yuqori Bu odatda quyidagicha ifodalanadi Dobson birliklari; qisqartirilgan "DU". Ozonning eng sezilarli pasayishi pastki stratosferaga to'g'ri keldi. Ustundagi ozonning belgilangan pasayishi Antarktika 1970 yillarning boshlariga nisbatan bahor va yozning boshlari kabi asboblar yordamida kuzatilgan Jami ozon xaritalash spektrometri (TOMS).[15]
Antarktida ustidan avstral (janubiy yarim shar) bahorida kuzatilgan va 1985 yilda birinchi marta xabar qilingan ozon kolonnasida 70 foizgacha pasayishlar davom etmoqda (Farman va boshqalar). Antarktidadagi umumiy ustunli ozon sentyabr va oktyabr oylarida 1990-yillardan beri ozon teshigidan oldingi ko'rsatkichlarga nisbatan 40-50 foizga past bo'lib qoldi.[1] "Davolash" ga bosqichma-bosqich tendentsiya haqida 2016 yilda xabar berilgan.[16] 2017 yilda NASA ozon teshigi 1988 yildan beri eng zaif bo'lganligini e'lon qildi, chunki iliq stratosfera sharoitlari mavjud edi. Taxminan 2070 yilda tiklanishi kutilmoqda.[17]
Yo'qotilgan miqdor yildan yilga o'zgarib turadi Arktika Antarktidaga qaraganda. Arktikaning eng katta pasayishi qishda va bahorda bo'lib, stratosfera eng sovuq bo'lganida 30 foizga etadi.
Qutbli stratosfera bulutlarida (PSC) sodir bo'ladigan reaktsiyalar ozon qatlamini kuchaytirishda muhim rol o'ynaydi.[18] PSClar Arktika va Antarktika stratosferasining keskin sovuqlarida osonroq shakllanadi. Shuning uchun ozon teshiklari Antarktida ustida birinchi bo'lib hosil bo'lgan va chuqurroqdir. Dastlabki modellar PSClarni hisobga olmadilar va asta-sekin global pasayishni bashorat qildilar, shuning uchun to'satdan Antarktika ozon teshigi ko'plab olimlar uchun ajablanib bo'ldi.[19][20][21]
Teshiklardan ko'ra ozonning pasayishi haqida o'rta kengliklarda gapirish to'g'riroq. Jami ustunli ozon o'rta kengliklarda 1980-1996 yillarda 1980 yilgacha bo'lgan ko'rsatkichlardan pastroq pasaygan. Keyinchalik shimoliy o'rta kengliklarda u qoidalar kuchga kirganligi va stratosferadagi xlor miqdori kamayganligi sababli 1996 yildan 2009 yilgacha minimal qiymatdan taxminan ikki foizga oshdi. Janubiy yarim sharning o'rta kengliklarida o'sha davr mobaynida umumiy ozon doimiy bo'lib qoldi. Tropik mintaqada sezilarli tendentsiyalar mavjud emas, asosan, tarkibida halogen bo'lgan birikmalar tropik kengliklarda xlor va brom atomlarini parchalashga va ajratishga ulgurmagan.[1][22]
1991 yilda Mt.ning otilishi bilan kuzatilganidek, katta vulkanik otilishlar ozonni emiruvchi ta'sirini notekis bo'lsada ko'rsatgan. Filippindagi Pinatubo.[23]
Ozonning emirilishi, shuningdek, stratosfera va yuqori troposfera haroratining pasayishining ko'pini tushuntiradi.[24][25] Stratosfera issiqligining manbai ultrabinafsha nurlanishining ozon tomonidan yutilishidir, shuning uchun ozonning sovishi sovutishga olib keladi. Ayrim stratosfera sovutishining oshishi bilan ham taxmin qilinadi issiqxona gazlari kabi CO
2 va CFClarning o'zi; ammo ozon ta'sirida sovutish dominant bo'lib ko'rinadi.[26]
Ozon sathini bashorat qilish qiyinligicha qolmoqda, ammo modellarning kuzatilgan qiymatlar bashoratining aniqligi va turli xil modellashtirish texnikalari o'rtasidagi kelishuv barqaror ravishda oshdi.[1] Jahon meteorologik tashkiloti Ozonni global tadqiq qilish va monitoring qilish loyihasi - 44-sonli ma'ruza Monreal protokoli foydasiga qat'iy chiqmoqda, ammo UNEP 1994 yil bahosi 1994-1997 yillar uchun ozon yo'qotilishini yuqori baholagan.[27]
Atmosferadagi birikmalar
Xloroflorokarbonatlar (OFK) va boshqa halogenlangan ozonni emiruvchi moddalar (ODS) asosan texnogen kimyoviy ozon qatlami uchun javobgardir. Stratosferadagi samarali halogenlarning (xlor va brom) umumiy miqdorini hisoblash mumkin va ular ekvivalent samarali stratosfera xlor (EESC).[28]
Sovutgich sifatida CFClar tomonidan ixtiro qilingan Tomas Midgli, kichik 1930-yillarda.[29] Ular ishlatilgan havo sovutish va sovutish moslamalari aerosol purkagichlari 1970-yillarga qadar va nozik elektron uskunalarni tozalash jarayonida. Ular ba'zi kimyoviy jarayonlarning yon mahsuloti sifatida ham uchraydi. Ushbu birikmalar uchun hech qachon muhim tabiiy manbalar aniqlanmagan - ularning atmosferada mavjudligi deyarli butun inson ishlab chiqarishi bilan bog'liq. Yuqorida ta'kidlab o'tilganidek, ozonni emiruvchi bunday kimyoviy moddalar stratosferaga etib kelganida, ular xlor atomlarini chiqarish uchun ultrabinafsha nurlari bilan ajralib chiqadi. Xlor atomlari a katalizator va ularning har biri stratosferadan chiqarilishidan oldin o'n minglab ozon molekulalarini parchalashi mumkin. CFC molekulalarining uzoq umrini hisobga olgan holda, tiklanish vaqtlari o'nlab yillar davomida o'lchanadi. Hisob-kitoblarga ko'ra, CFC molekulasi er sathidan atmosferaning yuqori qatlamiga o'tishi uchun o'rtacha besh yildan etti yilgacha davom etadi va u o'sha davrda yuz minggacha ozon molekulalarini yo'q qilib, u erda bir asr atrofida turishi mumkin.[30][tekshirish kerak ]
1,1,1-Trikloro-2,2,2-trifloroetan, shuningdek, CFC-113a nomi bilan tanilgan, Sharqiy Angliya universiteti jamoasi tomonidan atmosferada yangi kashf etilgan to'rtta kimyoviy kimyoviy moddalardan biridir. CFC-113a yagona ma'lum CFC uning atmosferasida ko'pligi hali ham o'sib bormoqda. Uning manbai sir bo'lib qolmoqda, ammo ba'zilar noqonuniy ishlab chiqarishda gumon qilishmoqda. CFC-113a 1960 yildan beri tinimsiz to'planib kelayotganga o'xshaydi. 2010-2012 yillarda gaz chiqindilari 45 foizga ko'tarildi.[31][32]
Xalqaro tadqiqotchilar guruhi tomonidan chop etilgan tadqiqot Tabiat 2013 yildan beri asosan Xitoyning shimoliy-sharqidan chiqadigan chiqindilar atmosferaga ko'p miqdordagi taqiqlangan Xloroflorokarbon-11 (CFC-11) kimyoviy moddasini chiqardi. Olimlarning hisob-kitoblariga ko'ra, ushbu CFC-11 chiqindilari sayyoramizning ozon teshigining tiklanishini o'n yilga kechiktiradi.[33][34][35]
Kompyuter modellashtirish
Olimlar ozon qatlamini inson tomonidan yaratilgan (antropogen ) kuzatuv ma'lumotlarini kompyuter modellari bilan birlashtirib, CFC dan halogen birikmalar. Ushbu murakkab kimyoviy transport modellari (masalan, SLIMCAT, Mollyuskalar —Stratosferaning kimyoviy lagranj modeli) kimyoviy moddalar va meteorologik maydonlarning o'lchovlarini kimyoviy reaksiya tezligi konstantalari bilan birlashtirish orqali ishlaydi. Ular CFCni keltirib chiqaradigan asosiy kimyoviy reaktsiyalar va transport jarayonlarini aniqlaydilar fotoliz ozon bilan aloqa qiladigan mahsulotlar.
Ozon teshigi va uning sabablari
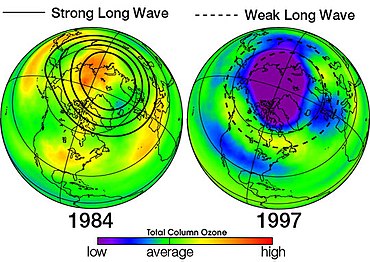
Antarktika ozon teshigi - bu Antarktika stratosferasining sohasi bo'lib, u erda yaqinda ozon darajasi 1975 yilgacha bo'lgan qiymatining 33 foizigacha pasaygan. Ozon teshigi Antarktika bahorida, sentyabrdan dekabr oyining boshigacha sodir bo'ladi, chunki kuchli g'arbiy shamollar materik atrofida aylanib, atmosfera konteynerini yaratadi. Shu doirada qutb girdobi, Antarktika bahorida pastki stratosfera ozonining 50 foizidan ko'prog'i yo'q qilinadi.[37]
Yuqorida aytib o'tilganidek, ozon yemirilishining asosiy sababi xlor o'z ichiga olgan manba gazlari (birinchi navbatda CFC va tegishli halokarbonlar) mavjudligidir. UV nurlari mavjud bo'lganda, bu gazlar ajralib chiqadi va xlor atomlarini chiqaradi, keyinchalik ular ozonni yo'q qilishni katalizatorga aylantiradi. Cl-katalizlangan ozon qatlami gaz fazasida sodir bo'lishi mumkin, ammo u mavjud bo'lganda keskin kuchayadi qutbli stratosfera bulutlari (PSClar).[38]
Ushbu qutbli stratosfera bulutlari qish paytida, qattiq sovuqda hosil bo'ladi. Polar qish qorong'i bo'lib, uch oylik quyosh nurlanishisiz (quyosh nurlari bo'lmagan) iborat. Quyosh nurlarining etishmasligi haroratning pasayishiga yordam beradi va qutbli girdob havoni ushlaydi va sovutadi. Harorat -80 ° C atrofida yoki pastda ko'tariladi. Ushbu past harorat bulut zarralarini hosil qiladi. PSC bulutlarining uch turi mavjud - nitrat kislota trihidratli bulutlar, asta-sekin soviydigan suvli muzli bulutlar va tez soviydigan suvli (muzli) bulutlar - kimyoviy reaktsiyalar uchun sirtlarni ta'minlaydi, ularning mahsulotlari bahorda ozon halokatiga olib keladi.[39]
The fotokimyoviy ishtirok etadigan jarayonlar murakkab, ammo yaxshi tushuniladi. Asosiy kuzatuv shundan iboratki, odatda, stratosferadagi xlorning ko'p qismi "rezervuar" birikmalarida, birinchi navbatda xlor nitratida (KLONO
2), shuningdek, HCl kabi barqaror oxirgi mahsulotlar. Oxirgi mahsulotlarning hosil bo'lishi, Cl ni ozon qatlamini yo'q qilish jarayonidan olib tashlaydi. Keyinchalik, 400 nm dan qisqa to'lqin uzunliklarida yorug'likni yutish orqali olish mumkin bo'lgan sobiq sekvestr Cl.[40] Antarktida qish va bahor davrida esa qutbli stratosfera buluti zarralari yuzasidagi reaktsiyalar ushbu "rezervuar" birikmalarini reaktiv erkin radikallarga (Cl va ClO) aylantiradi. Bulutlarni olib tashlash jarayoni YOQ
2 stratosferadan PSC zarralaridagi azot kislotasiga aylantirish orqali, keyinchalik cho'kindi jinslar natijasida yo'qoladigan denitrifikatsiya deyiladi. Bu yangi hosil bo'lgan ClO ning qayta konvertatsiya qilinishini oldini oladi KLONO
2.
Ozonning parchalanishida quyosh nurlarining roli Antarktida ozon qatlami bahor davrida eng katta bo'lishining sababi hisoblanadi. Qish paytida, PSClar eng ko'p bo'lsa ham, kimyoviy reaktsiyalarni qo'zg'atish uchun ustunda yorug'lik bo'lmaydi. Biroq bahor paytida quyosh chiqadi, fotokimyoviy reaktsiyalarni haydash va qutbli stratosfera bulutlarini eritish uchun energiya beradi va teshik mexanizmini boshqaradigan sezilarli ClO ni chiqaradi. Bahorning oxiri yaqinida haroratning ko'tarilishi dekabrning o'rtalarida girdobni buzadi. Issiq, ozon va YOQ
2- boylikli havo quyi kengliklardan kirib keladi, PSClar vayron bo'ladi, kuchaygan ozon qatlami jarayoni to'xtaydi va ozon teshigi yopiladi.[41]
Yo'q qilinadigan ozonning katta qismi, asosan, yuqori stratosferada sodir bo'ladigan bir hil gaz-fazali reaktsiyalar orqali ozonning ozayib ketishidan farqli o'laroq, pastki stratosferada joylashgan.[42]
Ozon qatlamining yemirilishiga qiziqish
Ozonning buzilishi kabi murakkab masalalar bo'yicha jamoatchilikda noto'g'ri tushunchalar va tushunmovchiliklar tez-tez uchraydi. Jamiyatning cheklangan ilmiy bilimlari global isish haqida chalkashliklarga olib keldi[43] yoki global isishni "ozon teshigi" ning bir qismi sifatida qabul qilish.[44]Dastlab, klassik yashil nodavlat notijorat tashkilotlari tashviqot uchun CFC tükenmesinden foydalanishdan bosh tortishdi, chunki bu mavzu juda murakkab edi.[45] Ular ancha keyin faollashdilar, masalan. sobiq Sharqiy Germaniya kompaniyasi tomonidan ishlab chiqarilgan CFC-bo'lmagan muzlatgichni Greenpeace-ning qo'llab-quvvatlashida VEB dkk Scharfenstein.[45][46]
CFC munozarasida ishlatiladigan metafora (ozon qalqoni, ozon teshigi) ilmiy ma'noda "aniq" emas. "Ozon teshigi" ko'proq depressiya, kamroq "old oynadagi teshik". Ozon qatlam orqali yo'qolib ketmaydi, ozon qatlamining bir tekis "siyraklashishi" mavjud emas. Biroq, ular olim bo'lmaganlar va ularning tashvishlari bilan yaxshiroq rezonanslashdi.[47] Ozon teshigi "dolzarb masala" va yaqinda yuzaga keladigan xavf sifatida qaraldi[48] chunki oddiy odamlar terining saraton kasalligi, katarakt, o'simliklarga zarar etkazishi va okeanning fonik zonasida plankton populyatsiyasining kamayishi kabi jiddiy shaxsiy oqibatlaridan qo'rqishgan. Nafaqat siyosat darajasida, iqlim o'zgarishiga nisbatan ozonni tartibga solish jamoatchilik fikri bo'yicha ancha yaxshi natijalarga erishdi. Amerikaliklar qonunchilik kuchga kirgunga qadar aerozol purkagichlardan ixtiyoriy ravishda voz kechishdi, iqlim o'zgarishi esa shunga o'xshash tashvish va jamoatchilik harakatlariga erisha olmadi.[47] 1985 yilda katta miqdordagi "teshik" mavjudligini to'satdan aniqlash, matbuotda keng tarqaldi. Antarktidada ayniqsa ozonning tez pasayishi ilgari o'lchov xatosi sifatida qabul qilingan edi.[49] Ilmiy konsensus tartibga solinganidan keyin o'rnatildi.[45]
Antarktika ozon teshigi global ozonga nisbatan ozgina ta'sir ko'rsatsa-da, teshik jamoatchilikda katta qiziqish uyg'otdi, chunki:
- Ko'pchilik ozon teshiklari dunyoning boshqa mintaqalarida paydo bo'la boshlashidan xavotirda edi, ammo hozirgi kunga qadar yagona yirik miqyosdagi tükenme faqat Shimoliy qutb atrofida Arktika bahorida kuzatilgan kichik ozon "chuqurligi" dir. O'rta kengliklarda ozon kamaydi, ammo juda ozroq (pasayish taxminan 4-5 foiz).
- Agar stratosfera sharoitlari yanada og'irlashsa (sovuqroq harorat, ko'proq bulutlar, faol xlor), global ozon katta tezlikda pasayishi mumkin. Standart Global isish nazariya stratosfera sovishini bashorat qilmoqda.[50]
- Antarktika ozon teshigi har yili parchalanib ketganda, ozon bilan ishlangan havo yaqin mintaqalarga chiqib ketadi. Antarktida ozon teshigi parchalanganidan keyingi bir oyda Yangi Zelandiyada ozon darajasining 10 foizgacha pasayishi qayd etildi,[51] ultrabinafsha-B nurlanish intensivligi bilan 1970-yillardan beri 15 foizdan oshdi.[52][53]
Ozon qatlamining yemirilishining oqibatlari
Ozon qatlami singib ketganligi sababli UVB Quyoshdan keladigan ultrabinafsha nurlar, ozon qatlamining emirilishi sirtdagi UVB darajasini oshiradi (barchasi teng), bu shikastlanishga olib kelishi mumkin, shu jumladan teri saratoni. Bu Monreal protokoli uchun sabab bo'lgan. Stratosfera ozonining pasayishi CFC bilan yaxshi bog'langan va UVB sirtining ko'payganiga qaramay, ozon qatlamini odamlarda teri saratoni va ko'zning shikastlanishi bilan bog'liq bo'lgan to'g'ridan-to'g'ri kuzatuv dalillari mavjud emas. Bu qisman, chunki UVA, shuningdek, terining saraton kasalligining ayrim shakllariga aloqador bo'lgan, ozon o'zlashtirmaydi va vaqt o'tishi bilan turmush tarzi o'zgarishi statistikasini nazorat qilish deyarli mumkin emas. Ozonning emirilishi shamol naqshlariga ham ta'sir qilishi mumkin.[54]
UVning ko'payishi
Ozon, Yer atmosferasida ozchilikni tashkil etsa-da, UVB nurlanishining ko'p emirilishi uchun javobgardir. Ozon qatlami orqali kirib boradigan UVB nurlanish miqdori eksponent ravishda kamayadi qatlamning qalinligi va zichligi bilan. Stratosfera ozon darajasi pasayganda, UVB ning yuqori darajasi Yer yuziga etib boradi.[1][55] Daraxt halqalarida ultrabinafsha nurlari ta'sirida fenol hosil bo'lishi 1700 yillarning oxiriga qadar shimoliy kengliklarda ozon parchalanishi boshlanishiga to'g'ri keladi.[56]
2008 yil oktyabr oyida Ekvador kosmik agentligi HIPERION deb nomlangan hisobotni nashr etdi. Tadqiqotda Ekvadordagi er usti asboblari va so'nggi 28 yil ichida bir nechta mamlakatlarning 12 ta sun'iy yo'ldoshlari ma'lumotlari ishlatilgan va ekvatorial kengliklarga etib kelgan ultrabinafsha nurlanish kutilganidan ancha yuqori bo'lganligi aniqlandi. UV indekslari 24 dyuymgacha ko'tarilish Kito; The JSSV 11 ni haddan tashqari indeks va sog'liq uchun katta xavf deb biladi. Hisobot xulosasiga ko'ra, sayyoramizning o'rta kengliklari atrofidagi ozon darajasining pasayishi, bu hududlarda allaqachon katta aholiga xavf tug'dirmoqda.[57] Keyinchalik, CONIDA, Peru kosmik agentligi, o'z tadqiqotini nashr etdi, bu Ekvador tadqiqotlari bilan deyarli bir xil natijalarga olib keldi.
Biologik ta'sir
Ozon teshigiga oid asosiy jamoatchilik tashvishi sirtdagi ultrabinafsha nurlanishining inson salomatligiga ta'siri bo'ldi. Hozirgacha aksariyat joylarda ozon qatlamining buzilishi odatda bir necha foizni tashkil etdi va yuqorida ta'kidlab o'tilganidek, ko'plab kengliklarda sog'liqqa zarar etkazilishining bevosita dalili mavjud emas. Agar ozon teshigida kuzatilgan yuqori darajadagi tükenme darajasi butun dunyoda keng tarqalgan bo'lsa, bu ta'sir sezilarli darajada dramatik bo'lishi mumkin. Antarktida ustidagi ozon teshigi ba'zi hollarda ba'zi qismlarga ta'sir qiladigan darajada kattalashgan Avstraliya, Yangi Zelandiya, Chili, Argentina va Janubiy Afrika, ekologlar UB sirtining ko'payishi sezilarli bo'lishi mumkinligidan xavotirda edilar.[58]
Ozonning yo'q bo'lib ketishi ularning barchasini kattalashtiradi ultrabinafsha nurlarining inson salomatligiga ta'siri, ham ijobiy (D vitamini ishlab chiqarishni ham o'z ichiga oladi), ham salbiy (shu jumladan quyosh yonishi, teri saratoni va katarakt). Bundan tashqari, ultrabinafsha nurlarining ko'payishi troposfera ozonining ko'payishiga olib keladi, bu odamlar uchun sog'liq uchun xavflidir.[59]
Bazal va skuamöz hujayrali karsinomalar
Odamlarda teri saratonining eng keng tarqalgan shakllari, bazal va yassi hujayrali karsinomalar, UVB ta'siriga juda bog'liq. UVB ushbu saratonni keltirib chiqaradigan mexanizm yaxshi tushunilgan - UVB nurlanishining yutilishi DNK molekulasida pirimidin asoslarini hosil bo'lishiga olib keladi dimerlar, natijada DNK takrorlanganda transkripsiyada xatolar yuz beradi. Ushbu saraton kasalliklari nisbatan engil va kamdan-kam hollarda o'limga olib keladi, ammo skuamoz hujayrali karsinomani davolash ba'zan keng ko'lamli rekonstruktiv operatsiyani talab qiladi. Epidemiologik ma'lumotlarni hayvonlarni o'rganish natijalari bilan birlashtirib, olimlar uzoq muddatli stratosfera ozonining har bir foizga pasayishi ushbu saraton kasalligini 2 foizga oshirishini taxmin qilishdi.[60]
Xatarli melanoma
Teri saratonining yana bir shakli, xavfli melanoma, tashxis qo'yilgan holatlarning taxminan 15-20 foizida o'limga olib keladigan juda kam tarqalgan, ammo juda xavfli. Xatarli melanoma va ultrabinafsha ta'sirlanish o'rtasidagi munosabatlar hali to'liq tushunilmagan, ammo UVB ham, UVA ham ishtirok etgan ko'rinadi. Ushbu noaniqlik tufayli ozon qatlamining melanoma paydo bo'lishiga ta'sirini taxmin qilish qiyin. Bir tadqiqot shuni ko'rsatdiki, UVB nurlanishining 10 foizga ko'payishi erkaklar uchun melanomalarning 19 foizga va ayollar uchun 16 foizga ko'payishi bilan bog'liq.[61] Insonlarni o'rganish Punta Arenas, janubiy uchida Chili, etti yil davomida melanoma 56 foizga o'sdi va melanoma bo'lmagan teri saratoni 46 foizga o'sdi, shu bilan birga ozon pasayishi va UVB darajasi oshdi.[62]
Kortikal katarakt
Epidemiologik tadqiqotlar ta'sir qilishning taxminiy taxminlari va kataraktni baholashning turli usullaridan foydalangan holda, ko'zning kortikal kataraktasi va UVB ta'sirlanishiga bog'liqligini ko'rsatadi. UVB ta'sirida ko'zning ta'sirlanishini batafsil baholash Chesapeake Bay Watermen-da o'tkazilgan tadqiqotda o'tkazildi, bu erda o'rtacha yillik ko'z ta'sirining oshishi kortikal xiralik xavfining ortishi bilan bog'liq edi.[63] Ko'pincha oq tanli erkaklar ta'sir qiladigan ushbu guruhda kortikal xiraliklarni quyosh nurlari bilan bog'laydigan dalillar hozirgi kungacha eng kuchli bo'lgan. Ushbu natijalarga asoslanib, ozon qatlamining yo'q bo'lib ketishi 2050 yilga kelib yuz minglab qo'shimcha kataraktalarga olib kelishi taxmin qilinmoqda.[64]
Troposfera ozonining ko'payishi
Yuzaki ultrafioletning ko'payishi ortishiga olib keladi troposfera ozon. Er osti ozoni odatda sog'liq uchun xavfli deb tan olinadi, chunki ozon kuchli bo'lgani uchun zaharli hisoblanadi oksidlovchi xususiyatlari. Xavf ayniqsa yosh bolalar, qariyalar va astma yoki boshqa nafas olish qiyinlishuvi bo'lganlar uchun katta. Ayni paytda ozon er osti darajasida asosan ultrabinafsha nurlanish ta'sirida hosil bo'ladi yonish avtomobil chiqindilaridan chiqadigan gazlar.[65]
D vitamini ishlab chiqarishning ko'payishi
D vitamini ultrabinafsha nurlari bilan terida ishlab chiqariladi. Shunday qilib, yuqori UVB ta'sirida inson D vitamini etishmayotganlarda ko'payadi.[66] Yaqinda o'tkazilgan tadqiqotlar (birinchi navbatda, Monreal protokolidan beri) ko'plab odamlarda D vitaminining maqbul darajasidan kamroq ekanligini ko'rsatdi. Xususan, AQSh aholisida D vitaminining eng past to'rtdan bir qismi (<17,8 ng / ml) Milliy sog'liqni saqlash va ovqatlanishni o'rganish tadqiqotlari ma'lumotlaridan foydalanib, umumiy populyatsiyada barcha sabablarga ko'ra o'limning ko'payishi bilan bog'liq.[67] Qonda D vitamini darajasi 100 ng / ml dan yuqori bo'lsa, u qonda kaltsiyni haddan tashqari oshirib yuborishi va o'lim darajasi yuqori bo'lishi bilan bog'liq bo'lsa-da, organizmda quyosh nurlari D vitaminini organizm talablaridan ortiqcha ishlab chiqarishiga to'sqinlik qiluvchi mexanizmlar mavjud.[68]
Hayvonlarga ta'siri
Londondagi Zoologiya instituti olimlarining 2011 yil noyabr oyida bergan hisobotida shuni aniqladilar kitlar Kaliforniya sohillari yaqinida quyosh nurlari keskin ko'tarilishini ko'rsatdi va bu olimlar "ozon qatlamining yupqalashiga sabab bo'lishidan qo'rqishadi".[69] Tadqiqot natijasida Kaliforniya ko'rfazidagi 150 dan ortiq kitdan teri biopsiyalari suratga olingan va olingan va DNK ultrabinafsha nurlanishidan zararlanganda hosil bo'ladigan hujayralarga ega bo'lgan "odatda o'tkir va qattiq quyosh yonishi bilan bog'liq bo'lgan epidermal shikastlanishning keng tarqalgan dalillari" topilgan. Topilmalar shuni ko'rsatadiki, "ozon qatlamining pasayishi natijasida ultrabinafsha nurlar darajasining ko'tarilishi, kuzatilgan terining shikastlanishida aybdor, xuddi shu kabi so'nggi o'n yilliklarda inson terisining saraton kasalligi o'sib bormoqda".[70] Kitlardan tashqari itlar, mushuklar, qo'ylar va quruqlikdagi ekotizimlar kabi ko'plab boshqa hayvonlar ham UV-B nurlanishining salbiy ta'siriga duch kelmoqdalar.[71]
Ekinlarga ta'siri
UV nurlanishining ko'payishi ekinlarga ta'sir qilishi kutilmoqda. Kabi bir qator iqtisodiy ahamiyatga ega o'simlik turlari guruch, bog'liq siyanobakteriyalar saqlab qolish uchun ularning ildizlarida istiqomat qilish azot. Siyanobakteriyalar ultrabinafsha nurlanishiga sezgir va uning ko'payishi ta'sir qiladi.[72] "Ko'tarilgan ultrabinafsha nurlanishining ta'sirini kamaytirish yoki tiklash mexanizmlariga qaramay, o'simliklar UVB darajasining ko'payishiga moslashish qobiliyati cheklangan, shuning uchun o'simliklarning o'sishiga UVB nurlanishi bevosita ta'sir qilishi mumkin."[73]
O'simliklar hayotiga ta'siri
Ozon qatlamining yo'q bo'lib ketishi va ortiqcha UVB nurlanishiga yo'l qo'yilishi dastlab o'simlik DNKiga etkazilgan zararni ko'payishiga olib keladi. Hisobotlar shuni ko'rsatdiki, o'simliklar stratosfera ozonining pasayishiga o'xshash UVB nurlanishiga duchor bo'lganda, o'simlik balandligi yoki barg massasida sezilarli o'zgarishlar bo'lmadi, ammo o'q otish biomassasida va barglar maydonida ozgina pasayishda o'z ta'sirini ko'rsatdi.[74] Biroq, UVB nurlanishi II fotosistemaning kvant rentabelligini pasayishini ko'rsatdi.[75] UVB shikastlanishi faqat haddan tashqari ta'sir ostida yuzaga keladi va aksariyat o'simliklarda UVB singdiruvchi flavonoidlar mavjud bo'lib, ular mavjud bo'lgan radiatsiyaga moslashishga imkon beradi. Rivojlanish davomida nurlanish ta'sirida bo'lgan o'simliklarga fotosintez tizimlari buzilganidan ko'ra kattaroq barg maydoni bilan yorug'likni ushlab tura olmaslik ta'sir qiladi.[76] UVB nurlanishining zarari o'simliklarning o'ziga emas, balki turlarning o'zaro ta'sirida katta ahamiyatga ega.[77]
Davlat siyosati
KFKlarning ozon qatlamiga etkazgan zararining to'liq darajasi ma'lum emas va o'nlab yillar davomida ma'lum bo'lmaydi; ammo ozon ustunining sezilarli pasayishi allaqachon kuzatilgan. Monreal va Vena konvensiyalari ilmiy konsensus o'rnatilishidan yoki fan sohasidagi muhim noaniqliklar echimidan ancha oldin o'rnatilgan edi.[45] Odamlar ozon holatini nisbatan yaxshi tushungan, masalan. Ozon qalqoni yoki ozon teshigi foydali "ko'priklarni metafora bilan tushunish oson" edi.[47] Amerikaliklar aerozolli purkagichlardan o'z ixtiyori bilan voz kechishdi, natijada qonunchilik amal qilinishidan oldin ham sotish hajmi 50 foizga kamaydi.[47]
1976 yilgi hisobotidan so'ng Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi ishonchli ilmiy dalillar ozon qatlami gipotezasini qo'llab-quvvatlaydi degan xulosaga keldi[78] AQSh, Kanada, Shvetsiya, Daniya va Norvegiyani o'z ichiga olgan bir necha mamlakatlar aerozol purkagich qutilarida CFC foydalanishni bekor qilishga o'tdilar.[79] O'sha paytda bu keng qamrovli tartibga solish siyosatiga qaratilgan birinchi qadam sifatida qaraldi, ammo keyingi yillarda siyosiy omillarning birlashishi (halokarbon sanoatining doimiy qarshiligi va atrof-muhitga bo'lgan munosabatning umumiy o'zgarishi) tufayli bu yo'nalishdagi taraqqiyot sustlashdi. Reygan ma'muriyatining dastlabki ikki yilidagi tartibga solish) va ilmiy ishlanmalar (ozon qatlamining kattaligining dastlabki baholari haddan tashqari katta bo'lganligini ko'rsatuvchi keyingi Milliy akademiya baholari). DuPont ishlab chiqarish uchun juda muhim patent Freon edi muddati 1979 yilda tugaydi. Qo'shma Shtatlar 1978 yilda aerozol qutilarida CFClardan foydalanishni taqiqlagan.[79] Evropa hamjamiyati CFC-larni aerozolli purkagichlarda taqiqlash to'g'risidagi takliflarni rad etdi va AQShda CFC-lar sovutgich va platalarni tozalash uchun ishlatishda davom etdi. Dunyo bo'ylab CFC ishlab chiqarish AQSh aerozol taqiqidan keyin keskin pasayib ketdi, ammo 1986 yilga kelib deyarli 1976 yilga qaytdi.[79] 1993 yilda, DuPont Kanada CFC muassasasini yopdi.[80]
AQSh hukumatining munosabati 1983 yilda yana o'zgara boshladi, qachon Uilyam Ruckelshaus almashtirildi Anne M. Burford ma'muri sifatida Qo'shma Shtatlar atrof-muhitni muhofaza qilish agentligi. Rukelshaus va uning o'rnini bosuvchi Li Tomas davrida EPA halokarbonli qoidalarga xalqaro yondashishni talab qildi. 1985 yilda eng yirik CFC ishlab chiqaruvchilarining ko'pchiligini o'z ichiga olgan yigirma mamlakat imzoladi Ozon qatlamini himoya qilish to'g'risidagi Vena konventsiyasi Ozonni buzuvchi moddalar bo'yicha xalqaro qoidalar bo'yicha muzokaralar uchun asos yaratdi. O'sha yili Antarktika ozon teshigi kashf etilgani e'lon qilindi va bu jamoatchilik e'tiborini ushbu masalaga qayta tikladi. 1987 yilda 43 davlat vakillari imzo chekdilar Monreal protokoli. Ayni paytda, halokarbon sanoati o'z pozitsiyasini o'zgartirdi va CFC ishlab chiqarishni cheklash to'g'risidagi protokolni qo'llab-quvvatlashni boshladi. Biroq, bu siljish DuPontning evropalik hamkasblariga qaraganda tezroq harakat qilishi bilan notekis edi. DuPont terining saraton kasalligi bilan bog'liq sud harakatlaridan qo'rqqan bo'lishi mumkin, ayniqsa, EPA 1986 yilda AQShda keyingi 88 yil ichida qo'shimcha 40 million holat va 800 000 saraton kasalligidan o'lim kutilmoqda, deb da'vo qilgan.[81] Germaniya CFC sanoatini himoya qilishdan voz kechib, tartibga solish bo'yicha harakatlarni qo'llab-quvvatlashni boshlagandan so'ng, Evropa Ittifoqi o'z pozitsiyasini o'zgartirdi. Monreal protokoli imzolanganidan keyin ham Frantsiya va Buyuk Britaniyadagi hukumat va sanoat o'zlarining CFC ishlab chiqaradigan sohalarini himoya qilishga harakat qildilar.[82]
Monrealda ishtirokchilar CFC ishlab chiqarishni 1986 yil darajasida muzlatib qo'yishga va 1999 yilga kelib ishlab chiqarishni 50 foizga kamaytirishga kelishib oldilar.[79] Antarktidaga qilingan bir qator ilmiy ekspeditsiyalar ozon teshigi chindan ham inson tomonidan ishlab chiqarilgan organogalogenlardan xlor va brom ta'sirida bo'lganligi to'g'risida ishonchli dalillar keltirgandan so'ng, Monreal protokoli 1990 yilda Londonda bo'lib o'tgan uchrashuvda mustahkamlandi. Ishtirokchilar CFC va halonlarni butunlay yo'q qilishga kelishib oldilar (masalan, ba'zi bir "muhim" foydalanish uchun belgilangan juda oz miqdordan tashqari). astma inhalerlari ) 2000 yilgacha 5-moddadan tashqari mamlakatlarda va 2010 yilgacha 5-moddaga (kam rivojlangan) imzo chekuvchilar.[83] 1992 yilda Kopengagendagi yig'ilishda uni tugatish sanasi 1996 yilga ko'chirildi.[83] Xuddi shu uchrashuvda, bromid metil (MeBr), asosan qishloq xo'jaligi ishlab chiqarishida ishlatiladigan fumigant, boshqariladigan moddalar qatoriga qo'shildi. For all substances controlled under the protocol, phaseout schedules were delayed for less developed ('Article 5(1)') countries, and phaseout in these countries was supported by transfers of expertise, technology, and money from non-Article 5(1) Parties to the Protocol. Additionally, exemptions from the agreed schedules could be applied for under the Essential Use Exemption (EUE) process for substances other than methyl bromide and under the Critical Use Exemption (CUE) process for methyl bromide.[84][85]
Civil society including especially NGOs, played critical roles at all stages of policy development leading up to the Vienna Conference, the Montreal Protocol, and in assessing compliance afterwards.[86][87][88][89] The major companies claimed that no alternatives to HFC existed.[90] An ozone-safe hydrocarbon refrigerant was developed at a Hamburg technological institute in Germany, consisting of a mixture of the hydrocarbon gases propan va butan, and in 1992 came to the attention of the non-governmental organization (NGO) Greenpeace. Greenpeace called it "Greenfreeze ".[91][92] The NGO then worked successfully first with a small and struggling company to market an appliance beginning in Europe, then Asia and later Latin America, receiving a 1997 UNEP award.[93][94] By 1995, Germany had already made CFC refrigerators illegal.[94] Since 2004, corporations like Coca-Cola, Carlsberg, and IKEA have been forming a coalition to promote the ozone-safe Greenfreeze units. Production spread to companies like Electrolux, Bosch, and LG, with sales reaching some 300 million refrigerators by 2008.[93][95] In Latin America, a domestic Argentinian company began Greenfreeze production in 2003, while the giant Bosch in Brazil began a year later.[96][97] By 2013 it was being used by some 700 million refrigerators, making up about 40 percent of the market.[90] In the U.S., however, change has been much slower. To some extent, CFCs were being replaced by the less damaging hydrochlorofluorocarbons (HCFClar ), although concerns remain regarding HCFCs also. In some applications, hydrofluorocarbons (HFClar ) were being used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute at all to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a (R-134a ), which in the United States has largely replaced CFC-12 (R-12 ) in automobile air conditioners. In laboratory analytics (a former "essential" use) the ozone depleting substances can be replaced with various other solvents.[98] Chemical companies like Du Pont, whose representatives even disparaged Greenfreeze as "that German technology," maneuvered the EPA to block the technology in the U.S. until 2011.[99][100][101][102] Ben & Jerry's of Unilever and General Electric, spurred by Greenpeace, had expressed formal interest in 2008 which figured in the EPA's final approval.[93][103]
More recently, policy experts have advocated for efforts to link ozone protection efforts to climate protection efforts.[104][105] Many ODS are also greenhouse gases, some thousands of times more powerful agents of radiative forcing than carbon dioxide over the short and medium term. Thus policies protecting the ozone layer have had benefits in mitigating Iqlim o'zgarishi. In fact, the reduction of the radiative forcing due to ODS probably masked the true level of climate change effects of other greenhouse gases, and was responsible for the "slow down" of global warming from the mid-90s.[106][qo'shimcha ma'lumot (lar) kerak ] Bir maydondagi siyosat qarorlari, ikkinchisida atrof-muhitni yaxshilash xarajatlari va samaradorligiga ta'sir qiladi.
ODS requirements in the marine industry
The IMO has amended MARPOL Annex VI Regulation 12 regarding ozone depleting substances. As from July 1, 2010, all vessels where MARPOL Annex VI is applicable should have a list of equipment using ozone depleting substances. The list should include name of ODS, type and location of equipment, quantity in kg and date. All changes since that date should be recorded in an ODS Record book on board recording all intended or unintended releases to the atmosphere. Furthermore, new ODS supply or landing to shore facilities should be recorded as well.
Prospects of ozone depletion
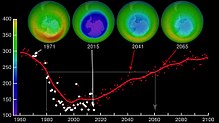

Since the adoption and strengthening of the Monreal protokoli has led to reductions in the emissions of CFCs, atmospheric concentrations of the most-significant compounds have been declining. These substances are being gradually removed from the atmosphere; since peaking in 1994, the Effective Equivalent Chlorine (EECl) level in the atmosphere had dropped about 10 percent by 2008. The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in brom -containing chemicals. The data suggest that substantial natural sources exist for atmospheric methyl bromide (CH
3Br).[1] The phase-out of CFCs means that azot oksidi (N
2O), which is not covered by the Montreal Protocol, has become the most highly emitted ozone-depleting substance and is expected to remain so throughout the 21st century.[107]
2005 yil IPCC review of ozone observations and model calculations concluded that the global amount of ozone has now approximately stabilized. Although considerable variability is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to begin to recover in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.[108]
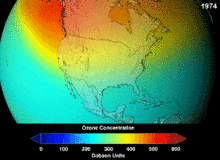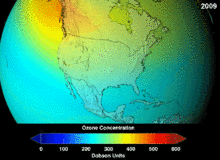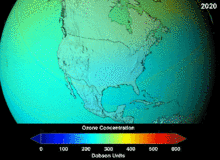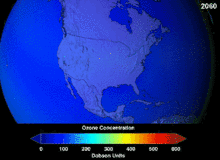
The Antarctic ozone hole is expected to continue for decades. Ozone concentrations in the lower stratosphere over Antarctica will increase by 5–10 percent by 2020 and return to pre-1980 levels by about 2060–2075. This is 10–25 years later than predicted in earlier assessments, because of revised estimates of atmospheric concentrations of ozone-depleting substances, including a larger predicted future usage in developing countries. Another factor that may prolong ozone depletion is the drawdown of nitrogen oxides from above the stratosphere due to changing wind patterns.[109] "Davolash" ga bosqichma-bosqich tendentsiya haqida 2016 yilda xabar berilgan.[16] In 2019, the ozone hole was at its smallest in the previous thirty years, due to the warmer polar stratosphere weakening the polar vortex.[110]
Tadqiqot tarixi
The basic physical and chemical processes that lead to the formation of an ozone layer in the Earth's stratosphere were discovered by Sidney Chapman in 1930. Short-wavelength UV radiation splits an oxygen (O
2) molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + O
3 → 2O
2. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance—it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is.
1970 yilda Pol Kruzzen pointed out that emissions of azot oksidi (N
2O), a stable, long-lived gas produced by soil bacteria, from the Earth's surface could affect the amount of azot oksidi (NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of o'g'itlar might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could affect the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from supersonic passenger aircraft, which would fly in the lower stratosphere, could also deplete the ozone layer. However, more recent analysis in 1995 by David W. Fahey, an atmospheric scientist at the Milliy okean va atmosfera boshqarmasi, found that the drop in ozone would be from 1–2 percent if a fleet of 500 supersonic passenger aircraft were operated.[111] This, Fahey expressed, would not be a showstopper for advanced supersonic passenger aircraft development.[112]
Rowland–Molina hypothesis
1974 yilda Frenk Shervud Roulend, Chemistry Professor at the University of California at Irvine, and his postdoctoral associate Mario J. Molina suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. Jeyms Lovelok had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like N
2O, the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing chlorine atoms. A year earlier, Richard Stolarski and Ralf Tsitseron at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by Maykl Makelroy va Stiven Vofsi da Garvard universiteti. Neither group, however, had realized that CFCs were a potentially large source of stratospheric chlorine—instead, they had been investigating the possible effects of HCl emissions from the Space Shuttle, which are very much smaller.
The Rowland–Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The Chair of the Board of DuPont was quoted as saying that ozone depletion theory is "a science fiction tale … a load of rubbish … utter nonsense".[113] Robert Abplanalp, the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of Irvin UC to complain about Rowland's public statements.[114] Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFCs and related compounds) and the chlorine reservoir species (HCl and ClONO
2) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone—its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the brominated organic compounds sifatida tanilgan halons, widely used in fire extinguishers, were a potentially large source of stratospheric bromine. 1976 yilda Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi released a report concluding that the ozone depletion hypothesis was strongly supported by the scientific evidence. In response the United States, Canada and Norway banned the use of CFCs in aerosol spray cans in 1978. Early estimates were that, if CFC production continued at 1977 levels, the total atmospheric ozone would after a century or so reach a steady state, 15 to 18 percent below normal levels. By 1984, when better evidence on the speed of critical reactions was available, this estimate was changed to 5 to 9 percent steady-state depletion.[115]
Crutzen, Molina, and Rowland were awarded the 1995 Kimyo bo'yicha Nobel mukofoti for their work on stratospheric ozone.
Antarctic ozone hole
The discovery of the Antarctic "ozone hole" by Britaniya Antarktika tadqiqotlari olimlar Farman, Gardiner va Shanklin (first reported in a paper in Tabiat 1985 yil may oyida[116]) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than anyone had anticipated.[49] Sun'iy yo'ldosh o'lchovlari (TOMS onbord Nimbus 7 ) showing massive depletion of ozone around the janubiy qutb were becoming available at the same time.[117] However, these were initially rejected as unreasonable by data quality control algorithms (they were filtered out as errors since the values were unexpectedly low); the ozone hole was detected only in satellite data when the raw data was reprocessed following evidence of ozone depletion in joyida kuzatishlar.[82] Qachon dasturiy ta'minot was rerun without the flags, the ozone hole was seen as far back as 1976.[118]
Syuzan Sulaymon, an atmospheric chemist at the Milliy okean va atmosfera boshqarmasi (NOAA), proposed that kimyoviy reaktsiyalar kuni polar stratospheric clouds (PSCs) in the cold Antarktika stratosfera caused a massive, though localized and seasonal, increase in the amount of xlor present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed when there are very low temperatures, as low as −80 °C, and early spring conditions. In such conditions the muz kristallari of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds, which can deplete ozone easily.
Bundan tashqari, qutb girdobi shakllangan Antarktida is very tight and the reaction occurring on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. Bu gipoteza was decisively confirmed, first by laboratoriya measurements and subsequently by direct measurements, from the ground and from high-altitude samolyotlar, of very high concentrations of xlor oksidi (ClO) in the Antarctic stratosphere.[119]
Alternative hypotheses, which had attributed the ozone hole to variations in solar UV nurlanishi or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.[120]
Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics.[22] These trends were confirmed by satellite measurements. As a consequence, the major halocarbon-producing nations agreed to phase out production of CFCs, halons, and related compounds, a process that was completed in 1996.
1981 yildan beri Birlashgan Millatlar Tashkilotining Atrof-muhit dasturi, under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the Scientific Assessment of Ozone Depletion, based on satellite measurements. The 2007 report showed that the hole in the ozone layer was recovering and the smallest it had been for about a decade.[121]The 2010 report found, "Over the past decade, global ozone and ozone in the Arctic and Antarctic regions is no longer decreasing but is not yet increasing. The ozone layer outside the Polar regions is projected to recover to its pre-1980 levels some time before the middle of this century. In contrast, the springtime ozone hole over the Antarctic is expected to recover much later."[122]2012 yilda, NOAA va NASA reported "Warmer air temperatures high above the Antarctic led to the second smallest season ozone hole in 20 years averaging 17.9 million square kilometres. The hole reached its maximum size for the season on Sept 22, stretching to 21.2 million square kilometres."[123] A gradual trend toward "healing" was reported in 2016[16] and then in 2017.[124] It is reported that the recovery signal is evident even in the ozone loss saturation altitudes.[125]
The hole in the Earth's ozone layer over the South Pole has affected atmospheric circulation in the Southern Hemisphere all the way to the equator.[126] The ozone hole has influenced atmospheric circulation all the way to the tropics and increased rainfall at low, subtropical latitudes in the Southern Hemisphere.
Arctic ozone "mini-hole"
On March 3, 2005, the journal Tabiat[127] published an article linking 2004's unusually large Arctic ozone hole to solar wind activity.
On March 15, 2011, a record ozone layer loss was observed, with about half of the ozone present over the Arctic having been destroyed.[128][129][130] The change was attributed to increasingly cold winters in the Arctic stratosphere at an altitude of approximately 20 km (12 mi), a change associated with global warming in a relationship that is still under investigation.[129] By March 25, the ozone loss had become the largest compared to that observed in all previous winters with the possibility that it would become an ozone hole.[131] This would require that the quantities of ozone to fall below 200 Dobson units, from the 250 recorded over central Siberia.[131] It is predicted that the thinning layer would affect parts of Scandinavia and Eastern Europe on March 30–31.[131]
On October 2, 2011, a study was published in the journal Tabiat, which said that between December 2010 and March 2011 up to 80 percent of the ozone in the atmosphere at about 20 kilometres (12 mi) above the surface was destroyed.[132] The level of ozone depletion was severe enough that scientists said it could be compared to the ozone hole that forms over Antarctica every winter.[132] According to the study, "for the first time, sufficient loss occurred to reasonably be described as an Arctic ozone hole."[132] The study analyzed data from the Aura va CALIPSO satellites, and determined that the larger-than-normal ozone loss was due to an unusually long period of cold weather in the Arctic, some 30 days more than typical, which allowed for more ozone-destroying chlorine compounds to be created.[133] According to Lamont Poole, a co-author of the study, cloud and aerosol particles on which the chlorine compounds are found "were abundant in the Arctic until mid March 2011—much later than usual—with average amounts at some altitudes similar to those observed in the Antarctic, and dramatically larger than the near-zero values seen in March in most Arctic winters".[133]
In 2013, researchers analyzed the data and found the 2010–11 Arctic event did not reach the ozone depletion levels to classify as a true hole. A hole in the ozone is generally classified as 220 Dobson units or lower;[134] the Arctic hole did not approach that low level.[135][136] It has since been classified as a "mini-hole."[137]
Following the ozone depletion in 1997 and 2011, a 90% drop in ozone was measured by weather balloons over the Arctic in March 2020, as they normally recorded 3.5 parts per million of ozone, compared to only around 0.3 parts per million lastly, due to cold temperatures ever recorded since 1979, and a strong polar girdob which allowed chemicals, including chlorine and bromine, to gnaw away.[138]
A rare hole, the result of unusually low temperatures in the atmosphere above the north pole, was studied in 2020.[139][140]
Tibet ozone hole
As winters that are colder are more affected, at times there is an ozone hole over Tibet. In 2006, a 2.5 million kvadrat kilometr ozone hole was detected over Tibet.[141] Also again in 2011 an ozone hole appeared over mountainous regions of Tibet, Shinjon, Tsinxay va Hindu Kush, along with an unprecedented hole over the Arctic, though the Tibet one is far less intense than the ones over the Arctic or Antarctic.[142]
Potential depletion by storm clouds
Research in 2012 showed that the same process that produces the ozone hole over Antarctica occurs over summer storm clouds in the United States, and thus may be destroying ozone there as well.[143][144]
Ozonning yemirilishi va global isish
Boshqalar orasida, Robert Uotson had a role in the science assessment and in the regulation efforts of ozon qatlami va global isish.[45] Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment[45] to reach a consensus to provide the Siyosat ishlab chiqaruvchilar uchun IPCC qisqacha bayoni.
There are various areas of linkage between ozone depletion and global warming science:

- Xuddi shu CO
2 radiative forcing that produces global warming is expected to cool the stratosphere.[145] This cooling, in turn, is expected to produce a relative kattalashtirish; ko'paytirish in ozone (O
3) depletion in polar area and the frequency of ozone holes.[146] - Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes "observed stratospheric O
3 losses over the past two decades have caused a negative forcing of the surface-troposphere system"[24] of about −0.15 ± 0.10 vatt per square meter (W/m2).[108] - One of the strongest predictions of the greenhouse effect is that the stratosphere will cool.[145] Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the Milliy okean va atmosfera boshqarmasi "s Geophysical Fluid Dynamics Laboratory show that above 20 km (12 mi), the greenhouse gases dominate the cooling.[147]
- As noted under 'Public Policy', ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m2 of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.[108]
- The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are currently being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using computational modeling methods to model ozone destruction, that accounted for 78 percent of the ozone destroyed. Further refinement of that model accounted for 89 percent of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of fossil fuels.)
In 2019, NASA reported that there was no significant relation between size of the ozone hole and the climate change.[6]
Noto'g'ri tushunchalar
CFC weight
Since CFC molecules are heavier than air (nitrogen or oxygen), it is commonly believed that the CFC molecules cannot reach the stratosphere in significant amount.[148] However, atmospheric gases are not sorted by weight; the forces of wind can fully mix the gases in the atmosphere. Lighter CFCs are evenly distributed throughout the turbosphere and reach the upper atmosphere,[149] although some of the heavier CFCs are not evenly distributed.[150]
Percentage of man-made chlorine

Another misconception is that "it is generally accepted that natural sources of tropospheric chlorine are four to five times larger than man-made ones." While this statement is strictly true, troposfera chlorine is irrelevant; bu stratosfera chlorine that affects ozone depletion. Chlorine from okean spreyi is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanlar than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant.[151] Only methyl chloride, which is one of these halocarbons, has a mainly natural source,[152] and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from manmade sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers[153] have shown that the contribution is not significant compared to that from CFCs.A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of Erebus tog'i on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.[153]
Nevertheless, a 2015 study[154] showed that the role of Erebus tog'i volcano in the Antarctic ozone depletion was probably underestimated. Asosida NCEP/NCAR reanalysis data over the last 35 years and by using the NOAA HYSPLIT trajectory model, researchers showed that Erebus volcano gas emissions (including vodorod xlorid (HCl)) can reach the Antarctic stratosphere via high-latitude cyclones and then the qutb girdobi. Depending on Erebus volcano activity, the additional annual HCl mass entering the stratosphere from Erebus varies from 1.0 to 14.3 kt.
First observation
G.M.B. Dobson mentioned that when springtime ozone levels in the Antarctic over Xelli ko'rfazi were first measured in 1956, he was surprised to find that they were ~320 DU, or about 150 DU below spring Arctic levels of ~450 DU. These were at that time the only known Antarctic ozone values available. What Dobson describes is essentially the boshlang'ich from which the ozone hole is measured: actual ozone hole values are in the 150–100 DU range.[155]
The discrepancy between the Arctic and Antarctic noted by Dobson was primarily a matter of timing: during the Arctic spring ozone levels rose smoothly, peaking in April, whereas in the Antarctic they stayed approximately constant during early spring, rising abruptly in November when the polar vortex broke down.
The behavior seen in the Antarctic ozone hole is completely different. Instead of staying constant, early springtime ozone levels suddenly drop from their already low winter values, by as much as 50 percent, and normal values are not reached again until December.[156]
Location of hole
Some people thought that the ozone hole should be above the sources of CFCs. However, CFCs are well mixed globally in the troposfera va stratosfera. The reason for occurrence of the ozone hole above Antarctica is not because there are more CFCs concentrated but because the low temperatures help form polar stratospheric clouds.[157] In fact, there are findings of significant and localized "ozone holes" above other parts of the earth, like above Central Asia.[158]
World Ozone Day
1994 yilda Birlashgan Millatlar Tashkilotining Bosh assambleyasi voted to designate September 16 as the Xalqaro ozon qatlamini saqlash kuni, or "World Ozone Day",[159] to commemorate the signing of the Monreal protokoli[160] on that date in 1987.[161]
Shuningdek qarang
Adabiyotlar
- ^ a b v d e f "Twenty Questions and Answers About the Ozone Layer" (PDF). Ozon qatlamini ilmiy baholash: 2010 yil. Jahon meteorologiya tashkiloti. 2011 yil. Olingan 13 mart, 2015.
- ^ Andino, Jean M. (October 21, 1999). "Chlorofluorocarbons (CFCs) are heavier than air, so how do scientists suppose that these chemicals reach the altitude of the ozone layer to adversely affect it ?". Ilmiy Amerika. 264: 68.
- ^ "Part III. The Science of the Ozone Hole". Olingan 5 mart, 2007.
- ^ Antara Banerjee; va boshq. (2020). "A pause in Southern Hemisphere circulation trends due to the Montreal Protocol". 579. Tabiat. 544-548 betlar. doi:10.1038/s41586-020-2120-4.
- ^ a b "The Antarctic Ozone Hole Will Recover". NASA. 2015 yil 4-iyun. Olingan 2017-08-05.
- ^ a b Bowden, John (2019-10-21). "Ozone hole shrinks to lowest size since 1982, unrelated to climate change: NASA". Tepalik. Olingan 2019-10-22.
- ^ Ansari, Talal (October 23, 2019). "Ozone Hole Above Antarctica Shrinks to Smallest Size on Record" - www.wsj.com orqali.
- ^ Ciaccia, Chris; News, Fox (October 22, 2019). "Antarctic ozone hole shrinks to smallest size on record due to 'rare event'".
- ^ "Ozon teshigi - Ozon qatlamini buzadigan moddalarga oid Monreal protokoli". Theozonehole.com. 16 sentyabr 1987 yil. Olingan 2019-05-15.
- ^ "Xalqaro Ozon qatlamini saqlash kuni - 16 sentyabr". www.un.org. Olingan 2019-05-15.
- ^ "World of Change: Antarctic Ozone Hole". earthobservatory.nasa.gov. 2009-06-01. Olingan 2020-06-26.
- ^ Newman, Paul A. "Chapter 5: Stratospheric Photochemistry Section 4.2.8 ClX catalytic reactions". In Todaro, Richard M. (ed.). Stratosfera ozoni: elektron darslik. NASA Goddard Space Flight Center Atmospheric Chemistry and Dynamics Branch. Olingan 28 may, 2016.
- ^ "Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture)—Encyclopedia of Earth". Eoearth.org. Arxivlandi asl nusxasi on September 9, 2011.
- ^ Scientific Assessment of Ozone Depletion 2010, National Oceanic & Atmospheric Administration
- ^ "The Ozone Hole Tour: Part II. Recent Ozone Depletion". Kembrij universiteti. Olingan 28 mart, 2011.
- ^ a b v Sulaymon, S .; Ivy, D. J.; Kinnison, D.; Mills, M. J.; Neely Rr, 3rd; Schmidt, A. (June 30, 2016). "Antarktida ozon qatlamida davolovchi vujudga kelishi". Ilm-fan. 353 (6296): 269–74. Bibcode:2016Sci ... 353..269S. doi:10.1126 / science.aae0061. PMID 27365314.
- ^ Mersmann, Katy; Stein, Theo (November 2, 2017). "Warm Air Helped Make 2017 Ozone Hole Smallest Since 1988". nasa.gov. Olingan 31 dekabr, 2017.
- ^ U.S. EPA: Ozone Depletion. epa.gov
- ^ Zafar, A. Mannan; Müller, Rolf; Grooss, Jens-Uwe; Robrecht, Sabine; Vogel, Bärbel; Lehmann, Ralph (January 2018). "The relevance of reactions of the methyl peroxy radical (CH3O2) and methylhypochlorite (CH3OCl) for Antarctic chlorine activation and ozone loss" (PDF). Tellus B: Chemical and Physical Meteorology. 70 (1): 1507391. Bibcode:2018TellB..7007391Z. doi:10.1080/16000889.2018.1507391. ISSN 1600-0889. S2CID 106298119.
- ^ Son, Seok-Woo; Han, Bo-Reum; Garfinkel, Chaim I.; Kim, Seo-Yeon; Park, Rokjin; Abraham, N. Luke; Hideharu Akiyoshi; Archibald, Alexander T.; Butchart, N. (2018). "Tropospheric jet response to Antarctic ozone depletion: An update with Chemistry-Climate Model Initiative (CCMI) models". Atrof-muhitni o'rganish bo'yicha xatlar. 13 (5): 054024. Bibcode:2018ERL....13e4024S. doi:10.1088/1748-9326/aabf21. ISSN 1748-9326.
- ^ "Largest-ever Ozone Hole over Antarctica". earthobservatory.nasa.gov. 2000-09-11. Olingan 2018-11-26.
- ^ a b "Myth: Ozone Depletion Occurs Only In Antarctica". EPA. 2006 yil 28 iyun. Olingan 28 mart, 2011.
- ^ Self, Stephen, et al. (1996). "1991 yildagi Pinatubo portlashining atmosferaga ta'siri". USGS. Olingan 28 may, 2016.
- ^ a b "Climate Change 2001: Working Group I: The Scientific Basis". Iqlim o'zgarishi bo'yicha hukumatlararo hay'at Work Group I. 2001. pp. Chapter 6.4 Stratospheric Ozone. Arxivlandi asl nusxasi 2016 yil 3-iyun kuni. Olingan 28 may, 2016.
- ^ 2008 News, Briefs, and Features. NASA
- ^ "Climate Change 2013: The Physical Science Basis". UNEP. Olingan 28 may, 2016.
- ^ "Scientific Assessment of Ozone Depletion 1998 – Preface". US National Oceanic & Atmospheric Administration. Olingan 21 dekabr 2012.
- ^ Newman, P. A.; Daniel, J. S.; Waugh, D. W.; Nash, E. R. (2007). "A new formulation of equivalent effective stratospheric chlorine (EESC)" (PDF). Atmos. Kimyoviy. Fizika. 7 (17): 4537–52. doi:10.5194/acp-7-4537-2007.
- ^ Kauffman, G.B. (2005). "CFCs: On the 75th Anniversary of Their Introduction as Commercial Refrigerants by Thomas Midgley, Jr. (1889–1944)". Kimyoviy. Tarbiyachi. 10 (3): 217–226. doi:10.1333/s00897050916a.
- ^ "chlorofluorocarbons". Encyclopedia.com. Olingan 28 mart, 2011.
- ^ Laube, Yoxannes S.; Nyuland, Mayk J.; Xogan, Kristofer; Brenninkmeijer, Karl A. M.; Freyzer, Pol J.; Martinerie, Patrisiya; Oram, Devid E.; Rivz, Kler E .; Rokman, Tomas; Schwander, Jakob; Vitrant, Emmanuel; Sturges, William T. (March 9, 2014). "Atmosferada yangi aniqlangan ozonni emiruvchi moddalar" (PDF). Tabiatshunoslik. 7 (4): 266–269. Bibcode:2014NatGe...7..266L. doi:10.1038 / ngeo2109.
- ^ McGrath, Matt (2014-03-09). "Sirli yangi texnogen gazlar ozon qatlamiga tahdid solmoqda". BBC yangiliklari. Olingan 10 mart, 2014.
- ^ McGrath, Matt (2019-05-22). "China confirmed as source of rise in CFCs". BBC yangiliklari. Olingan 2020-04-08.
- ^ "China factories releasing thousands of tonnes of illegal CFC gases, study finds". Guardian. 2019-05-23. Olingan 2020-04-08.
- ^ Stoye2019-05-22T18:00:00+01:00, Emma. "China identified as source of unexpected rise in CFC emissions". Kimyo olami. Olingan 2020-04-08.
- ^ Nesh, Erik; Newman, Paul (September 19, 2001). "NASA Confirms Arctic Ozone Depletion Trigger". Image of the Day. NASA. Olingan 16 aprel, 2011.
- ^ Sparling, Brien (June 26, 2001). "Antarctic Ozone Hole". NASA Advanced Supercomputing Department. Archived from the original on April 3, 2009. Olingan 16 aprel, 2011.CS1 maint: yaroqsiz url (havola)
- ^ Parson, Robert (December 16, 1997). "Antarctic ozone-depletion FAQ, section 7". Faqs.org. Olingan 16 aprel, 2011.
- ^ Toon, Ouen B.; Turco, Richard P. (June 1991). "Polar Stratospheric Clouds and Ozone Depletion" (PDF). Ilmiy Amerika. 264 (6): 68–74. Bibcode:1991SciAm.264...68T. doi:10.1038/scientificamerican0691-68. Arxivlandi asl nusxasi (PDF) 2011 yil 25 fevralda. Olingan 16 aprel, 2011.
- ^ Sumi´nska-Ebersoldt; Lehmann, R .; Wegner, T.; Grooß, J.-U.; Hösen, E.; Weigel, R.; Frey, W.; Griessbach, S.; Mitev, V.; Emde, C .; Volk, C. M.; Borrmann, S.; Rex, M.; Stroh, F.; von Hobe, M. (July 2011). "ClOOCl photolysis at high solar zenith angles: analysis of the RECONCILE self-match flight". Atmos. Kimyoviy. Fizika. 12 (3): 1353–1365. Bibcode:2012ACP....12.1353S. doi:10.5194/acp-12-1353-2012.
- ^ "Ozone Facts: What is the Ozone Hole?". Ozone Hole Watch. NASA. 2009 yil 18-noyabr. Olingan 16 aprel, 2011.
- ^ Rowland, Frank Sherwood (May 29, 2006). "Stratospheric ozone depletion". Fil. Trans. R. Soc. B. 361 (1469): 769–790. doi:10.1098/rstb.2005.1783. PMC 1609402. PMID 16627294.
4. Free radical reactions for ozone removal: Reaction 4.1
- ^ Boyesa, Edward; Stanisstreeta, Martin (1992). "Students' perceptions of global warming". Xalqaro ekologik tadqiqotlar jurnali. 42 (4): 287–300. doi:10.1080/00207239208710804.
- ^ Compare Sheldon Ungar, 2000 and various web sites such as Gavin Schmidt 's realclimate complaint in Ozone depletion and global warming 2005 yoki UCS FAQ on the topic
- ^ a b v d e f Reiner Grundmann Technische Problemlösung, Verhandeln und umfassende Problemlösung, generic problem solving capability) Gesellschaftliche Kompleksität und kollektive Handlungsfähigkeit (Jamiyatning murakkabligi va jamoaviy harakat qobiliyati), ed. Shimank, U. (2000). Frankfurt / Main: Kampus, p.154-182 Maks Plank Gesellschaft-dagi kitobning qisqacha mazmuni Arxivlandi 2014-10-12 at the Orqaga qaytish mashinasi
- ^ Gunkel, Christoph (September 13, 2013). "Öko-Coup aus Ostdeutschland". Der Spiegel (nemis tilida). Olingan 4 sentyabr 2015.
- ^ a b v d Ungar, Sheldon (1 July 2000). "Knowledge, ignorance and the popular culture: climate change versus the ozone hole". Ilm-fanning jamoatchilik tushunchasi. 9 (3): 297–312. doi:10.1088/0963-6625/9/3/306. S2CID 7089937.
- ^ Grundmann, Reiner (May 14, 2007). "Iqlim o'zgarishi va bilim siyosati" (PDF). Atrof-muhit siyosati. 16 (3): 414–432. CiteSeerX 10.1.1.535.4984. doi:10.1080/09644010701251656. S2CID 153866225. Arxivlandi asl nusxasi (PDF) 2014 yil 26 avgustda.
- ^ a b Zehr, Stephen C. (1994). "Accounting for the Ozone Hole: Scientific Representations of an Anomaly and Prior Incorrect Claims in Public Settings". Sotsiologik chorak. 35 (4): 603–19. doi:10.1111/j.1533-8525.1994.tb00419.x. JSTOR 4121521.
- ^ "Climate Change 2001: Working Group I: The Scientific Basis". Iqlim o'zgarishi bo'yicha hukumatlararo hay'at Work Group I. 2001. pp. Chapter 9.3.2 Patterns of Future Climate Change. Arxivlandi asl nusxasi 2016 yil 3-iyun kuni. Olingan 28 may, 2016.
- ^ Muir, Patricia (March 6, 2008). "Stratospheric Ozone Depletion". Oregon shtat universiteti. Olingan 16 aprel, 2011.
- ^ "Long-term increase in summer UV radiation". NIWA. 1999-09-09. Olingan 4 dekabr, 2013.
- ^ McKenzie, Richard; Conner, Brian; Bodeker, Greg (September 10, 1999). "Increased Summertime UV Radiation in New Zealand in Response to Ozone Loss". Ilm-fan. 285 (5434): 1709–1711. doi:10.1126/science.285.5434.1709. PMID 10481002.
- ^ Banerjee, Antara (25 March 2020). "A pause in Southern Hemisphere circulation trends due to the Montreal Protocol". Tabiat. 579 (7800): 544–548. Bibcode:2020Natur.579..544B. doi:10.1038/s41586-020-2120-4. PMID 32214266. S2CID 214648481. Olingan 31 mart 2020.
- ^ "Health and Environmental Effects of Ozone Layer Depletion". EPA. 2013-02-15. Olingan 26 sentyabr, 2013.
- ^ "Reconstruction of Paleobehavior of Ozonosphere Based on Response to UV-B Radiation Effect in Dendrochronologic Signal" (PDF). Atmospheric Radiation Measurement, USA. Olingan 28 may, 2016.
- ^ The HIPERION Report (PDF) (Hisobot). Ecuadorian Civilian Space Agency. 2008 yil.
- ^ Lilley, Ray (October 5, 2000). "Ozone Hole Over City for First Time". Associated Press. Olingan 13 mart, 2015.
- ^ Bais, F.; Luca, R. M.; Bornman, J. F.; Williamson, C. E.; Sulzberger, B.; Austin, A. T.; Wilson, S. R.; Andrady, A. L.; Bernhard, G.; McKenzie, R. L.; Aucamp, P. J. (2018-02-14). "Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017". Photochemical & Photobiological Sciences. 17 (2): 127–179. doi:10.1039/c7pp90043k. ISSN 1474-905X. PMC 6155474. PMID 29404558.
- ^ de Gruijl, Frank R. (Summer 1995). "Impacts of a Projected Depletion of the Ozone Layer". Oqibatlari. 1 (2).
- ^ Fears, T. R.; Bird, C. C.; Guerry d, 4th; Sagebiel, R. W.; Gail, M. H.; Elder, D. E.; Halpern, A .; Holly, E. A.; Hartge, P.; Tucker, M. A. (2002). "Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk". Saraton kasalligi. 62 (14): 3992–6. PMID 12124332.
- ^ Abarca, J. F.; Casiccia, C. C. (December 2002). "Antarktika ozon teshigi ostida teri saratoni va ultrabinafsha-B nurlanishi: janubiy Chili, 1987-2000". Photodermatol Photoimmunol Photomed. 18 (6): 294–302. doi:10.1034 / j.1600-0781.2002.02782.x. PMID 12535025. S2CID 25748826.
- ^ West, S. K.; Duncan, D. D.; Muñoz, B .; Rubin, G. S.; Fried, L. P.; Bandin-Rosh, K .; Schein, O. D. (1998). "Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project". JAMA. 280 (8): 714–8. doi:10.1001/jama.280.8.714. PMID 9728643.
- ^ Dobson, R. (2005). "Ozone depletion will bring big rise in number of cataracts". BMJ. 331 (7528): 1292–1295. doi:10.1136/bmj.331.7528.1292-d. PMC 1298891.
- ^ "Ozone: Good Up High, Bad Nearby" (PDF). EPA. Archived from the original on June 2, 2013. Olingan 13 mart, 2015.CS1 maint: yaroqsiz url (havola)
- ^ Webb, Ann R.; Engelsen, Ola (2006). "Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status". Fotokimyo va fotobiologiya. 82 (6): 1697–1703. doi:10.1111/j.1751-1097.2006.tb09833.x. ISSN 1751-1097. PMID 16958558. S2CID 222102318.
- ^ Melamed, M. L.; Michos, E. D.; Post, W.; Astor, B. (2008). "25-hydroxyl Vitamin D Levels and the Risk of Mortality in the General Population". Arch. Stajyor. Med. 168 (15): 1629–37. doi:10.1001/archinte.168.15.1629. PMC 2677029. PMID 18695076.
- ^ Vieth R (1999). "D vitamini qo'shilishi, D-25 gidroksivitamin D konsentratsiyasi va xavfsizligi". Am. J. klinikasi. Nutr. 69 (5): 842–56. doi:10.1093 / ajcn / 69.5.842. PMID 10232622.
- ^ "Sunburned whales: Troubling environment news of the week". Washington Post. BlogPost (blog). 2010 yil 11-noyabr. Olingan 28 mart, 2011.
- ^ Thomas, Abbie (November 10, 2010). "Whales showing more sun damage". Abc.net.au. Olingan 28 mart, 2011.
- ^ Mayer, S. J. (1992-08-08). "Stratospheric ozone depletion and animal health". Veterinariya qaydlari. 131 (6): 120–122. doi:10.1136/vr.131.6.120. ISSN 0042-4900. PMID 1529513. S2CID 22177257.
- ^ Sinha, R. P.; Singh, S. C.; Häder, D. P. (1999). "Photoecophysiology of cyanobacteria". Recent Research Developments in Photochemistry and Photobiology. 3: 91–101.
- ^ "Health and Environmental Effects of Ozone Layer In Plants". U.S Environmental Protection Agency. 2013-02-15. Olingan 12-noyabr, 2013.
- ^ Searles, Peter S.; Flint, Stephan D.; Caldwell, Martyn M. (2001-03-01). "A meta-analysis of plant field studies simulating stratospheric ozone depletion". Ekologiya. 127 (1): 1–10. Bibcode:2001Oecol.127....1S. doi:10.1007/s004420000592. ISSN 1432-1939. PMID 28547159. S2CID 7049908.
- ^ Xiong, Fusheng S.; Kun, Tomas A. (2001-02-01). "Ozonning ozayib ketishi paytida quyosh ultrabinafsha-B nurlanishining antarktika qon tomir o'simliklarining fotosintezi va biomassasini ishlab chiqarishga ta'siri". O'simliklar fiziologiyasi. 125 (2): 738–751. doi:10.1104 / s.125.2.738. ISSN 0032-0889. PMC 64875. PMID 11161031.
- ^ Allen, Damian J.; Nogues, Salvador; Beyker, Nil R. (1998-11-01). "Ozonning emirilishi va UV-B nurlanishining ko'payishi: fotosintez uchun haqiqiy xavf bormi?". Eksperimental botanika jurnali. 49 (328): 1775–1788. doi:10.1093 / jxb / 49.328.1775. ISSN 0022-0957.
- ^ Byorn, Lars Olof (1996-12-01). "Ozonning emirilishi va UV ‐ B ning er usti ekotizimlariga ta'siri". Xalqaro ekologik tadqiqotlar jurnali. 51 (3): 217–243. doi:10.1080/00207239608711082. ISSN 0020-7233.
- ^ Milliy fanlar akademiyasi (1976). Galokarbonlar, stratosfera ozoniga ta'siri. Vashington, DC. ISBN 9780309025324. Olingan 28 may, 2016.
- ^ a b v d Morrisette, Piter M. (1989). "Stratosfera ozon qatlamining pasayishiga qarshi siyosat evolyutsiyasi". Natural Resources Journal. 29: 793–820. Olingan 20 aprel, 2010.
- ^ Savchuk, Artur R. (1994 yil 19-dekabr). "Issiqxona gazlari chiqindilarini kamaytirish bo'yicha ixtiyoriy tashabbuslar", "Arxivlangan nusxa" (PDF). Arxivlandi asl nusxasi (PDF) 2011 yil 6-iyulda. Olingan 2010-06-03.CS1 maint: nom sifatida arxivlangan nusxa (havola). DuPont Canada Inc.
- ^ Shabekoff, Filipp (1986 yil 5-noyabr). "AQSh hisobotida ozon yo'qotilishi bilan teri saratoni ko'payishi bashorat qilingan". The New York Times. p. A1. Olingan 10 yanvar, 2013.
- ^ a b Grundmann, Reyner (2001). Transmilliy ekologik siyosat: ozon qatlami. Nyu-York: Routledge. ISBN 978-0-415-22423-9.
- ^ a b "Monreal protokoliga o'zgartirishlar | Ozon qatlamini himoya qilish | AQSh EPA". Epa.gov. 2006 yil 28 iyun. Olingan 28 mart, 2011.
- ^ Gareau, Brian J. (2010). "Monreal protokolida kechiktirilgan metil bromidni bekor qilishga qarshi CFC ning muvaffaqiyatli bekor qilinishini tanqidiy ko'rib chiqish". Xalqaro ekologik shartnomalar: siyosat, huquq va iqtisod. 10 (3): 209–231. doi:10.1007 / s10784-010-9120-z. S2CID 153692785.
- ^ Dekanio, Stiven J.; Norman, Ketrin S. (2005 yil iyul). "Bromid metilini" tanqidiy foydalanish "Monreal protokoli bo'yicha iqtisodiyoti". Zamonaviy iqtisodiy siyosat. 23 (3): 376–393. doi:10.1093 / cep / byi028.
- ^ Sarma, K. Madhava "Ozon qatlamini muhofaza qilish bo'yicha ko'p tomonlama ekologik bitimlarga rioya qilish" Ulrich Beyerlin va boshq. Leyden: Martinus Nixof 2006 yildagi ko'p tomonlama ekologik bitimlarga muvofiqligini ta'minlash
- ^ Mate, Jon (2001). "Farq yaratish: Greenpeace ozon kampaniyasining amaliy tadqiqoti". Evropa hamjamiyati va xalqaro ekologik huquqni ko'rib chiqish. 10 (2): 190–198. doi:10.1111/1467-9388.00275.
- ^ Currie, Duncan E.J. (2005) Tullio Treves va boshqalarda "Greenpeace International tajribasi". (tahr.) Fuqarolik jamiyati, xalqaro sudlar va muvofiqlik organlari Gaaga, Gollandiya: TMC Asser.
- ^ Benedik, Richard Elliot (1991) Ozon diplomatiyasi. Kembrij, MA: Garvard universiteti.
- ^ a b "Tug'ilgan kuningiz bilan, Greenfreeze!". Greenpeace International. Olingan 28 may, 2016.
- ^ Stafford, Edvin R.; Xartman, Keti L.; Liang, Ying (2016-10-10). "Xitoyda ekologik innovatsion diffuziyani qo'zg'atadigan kuchlar: Greenfriz ishi" (PDF). Biznes ufqlari. 46 (2): 47–56. doi:10.1016 / S0007-6813 (03) 00009-0. Arxivlandi asl nusxasi (PDF) 2016-10-10 kunlari.
- ^ "AQShga iqlimga mos Greenfrizerlar keladi". NBC Nyu-York. Olingan 28 may, 2016.
- ^ a b v "Greenpeace USA". Greenpeace.org. 2015 yil 23 sentyabr. Olingan 27 sentyabr, 2015.
- ^ a b "Greenfreeze: maishiy sovutgichdagi inqilob". Ecomall.com. 1995 yil 1-yanvar. Olingan 28 may, 2016.
- ^ "Tabiiy sovutgichlar - biznes". Greenpeace International. Olingan 28 may, 2016.
- ^ "La Historia del" Grinfriz"". Ilustrados!. Olingan 27 sentyabr, 2015.
- ^ "Lanzan la primera de las" Propuestas Greenpeace ": la heladera" Greenfreeze "| Greenpeace Argentina". Greenpeace.org. Olingan 27 sentyabr, 2015.
- ^ "Laboratoriyalarda ozon buzadigan moddalardan foydalanish. TemaNord 516/2003" (PDF). Norden.org. 2003 yil 1-yanvar. Asl nusxasidan arxivlangan 2008 yil 27 fevral. Olingan 28 mart, 2011.CS1 maint: yaroqsiz url (havola)
- ^ "Der Greenfreeze - endlich in den USA angekommen". Greenpeace. Olingan 28 may, 2016.
- ^ "Discurso de Frank Guggenheim no lançamento do Greenfreeze". Brasil. Olingan 28 may, 2016.
- ^ "SNAP dastur xronologiyasi | Alternativalar / SNAP | AQSh EPA". Epa.gov. 2014-10-15. Olingan 27 sentyabr, 2015.
- ^ "Greenfreeze F-Gas g'alabasi! AQShda yana yashilroq sovutgichlar qonuniy" Greenpeace AQSh. 14 dekabr 2011 yil. Arxivlangan asl nusxasi 2012 yil 29 yanvarda. Olingan 1 yanvar, 2018.
- ^ "GE kelajagini toza uyda sovutish uchun eshik ochmoqda" (Matbuot xabari). Asl nusxasidan arxivlangan 2011 yil 5 iyun. Olingan 24 avgust, 2014.CS1 maint: yaroqsiz url (havola)
- ^ Molina, M.; Zaelke, D .; Sarma, K. M .; Andersen, S. O .; Ramanatan, V .; Kaniaru, D. (2009). "Monreal protokoli va boshqa tartibga solish harakatlaridan foydalangan holda, iqlim o'zgarishi xavfini kamaytirish CO ning qisqarishini to'ldirish uchun2 emissiya ". Milliy fanlar akademiyasi materiallari. 106 (49): 20616–20621. Bibcode:2009PNAS..10620616M. doi:10.1073 / pnas.0902568106. PMC 2791591. PMID 19822751.
- ^ Norman, Ketrin; Dekanio, Stiven; Fan, Lin (2008). "Monreal protokoli 20-da: Iqlimni muhofaza qilish bilan birlashishning doimiy imkoniyatlari". Global atrof-muhit o'zgarishi. 18 (2): 330–340. doi:10.1016 / j.gloenvcha.2008.03.003.
- ^ Estrada, Fransisko (2013). "Yigirmanchi asrdagi harorat o'zgarishiga insonning turli xil ta'sirining statistik ravishda olingan hissalari". Tabiatshunoslik. 6 (12): 1050–1055. Bibcode:2013NatGe ... 6.1050E. doi:10.1038 / ngeo1999. hdl:2144/27169.
- ^ "NOAA tadqiqotida azot oksidi eng yaxshi ozonni emiradigan emissiya ko'rsatildi". Noaanews.noaa.gov. 2009 yil 27 avgust. Olingan 6 aprel, 2011.
- ^ a b v "Siyosat ishlab chiqaruvchilar uchun xulosa" (PDF). Ozon qatlami va global iqlim tizimini muhofaza qilish bo'yicha IPCC / TEAP maxsus hisoboti: gidroflorokarbonatlar va perflorokarbonlar bilan bog'liq muammolar. Kembrij: Iqlim o'zgarishi bo'yicha hukumatlararo panel uchun nashr etilgan [Muallif] Kembrij universiteti matbuoti. 2005 yil. ISBN 978-0-521-86336-0. Olingan 28 may, 2016.
- ^ Kanadaning SCISAT sun'iy yo'ldoshi 2006 yilda ozon qatlamining yo'q qilinishini tushuntiradi. Kanada kosmik agentligi. 2006 yil 6 oktyabr.
- ^ "Ozon teshigi yopiladi". Space Daily. Space Media Network. 12-noyabr, 2019-yil. Olingan 8 dekabr 2019.
- ^ Lipkin, Richard (1995 yil 7 oktyabr). SST emissiyasi stratosfera ozonini kamaytiradi. (2015 yilgacha 500 ta yangi ovozdan yuqori tezlikda harakatlanadigan transport samolyotlarining kiritilishi ozon qatlamini 1% gacha buzishi mumkin). Fan yangiliklari.
- ^ "Ovozdan tez ko'tariladigan samolyotlarning ko'payishi ozon U-2 samolyotining Concorde yo'llari uchun xavf tug'dirishi mumkin, chiqindi zarralarini o'rganmoqda". Baltimor quyoshi. Yangiliklar kuni. 1995 yil 8 oktyabr. Olingan 21 dekabr, 2012.
- ^ "Du Pont: 3D korporativ strategiyasida amaliy tadqiqotlar". Greenpeace. 1997. 2012 yil 6 aprelda asl nusxasidan arxivlangan.CS1 maint: yaroqsiz url (havola)
- ^ Roan, Sharon (1989) Ozon inqirozi: to'satdan global favqulodda vaziyatning 15 yillik evolyutsiyasi, Nyu-York: Wiley, p. 56 ISBN 0-471-52823-4
- ^ Stratosfera ozonini kamaytirish sabablari va ta'siri: yangilanish. Milliy tadqiqot kengashi. 1982. p. Xulosa, 3. doi:10.17226/319. ISBN 978-0-309-03248-3.
- ^ Farman, J. C.; Gardiner, B. G.; Shanklin, J. D. (1985). "Antarktidada umumiy ozonning katta yo'qotishlari mavsumiy ClO ni aniqlaydix/ YO'Qx o'zaro ta'sir ". Tabiat. 315 (6016): 207–210. Bibcode:1985 yil Natur.315..207F. doi:10.1038 / 315207a0. S2CID 4346468.
- ^ Bxartiya, Pavan Kumar; McPeters, Richard D. (2018). "Antarktika ozon teshigining kashf etilishi". Compends Rendus Geoscience. Elsevier BV. 350 (7): 335–340. doi:10.1016 / j.crte.2018.04.006. ISSN 1631-0713.
- ^ Tarix va siyosat kirish 2016 yil 30-sentyabr.
- ^ Sulaymon, P. M.; Konnor, B .; De Zafra, R. L .; Parish, A .; Barret, J .; Jaramillo, M. (1987). "Antarktida bahorgi stratosferada past balandliklarda xlor monoksitning yuqori konsentratsiyasi: Dunyoviy o'zgarish". Tabiat. 328 (6129): 411–413. Bibcode:1987 yil 328..411S. doi:10.1038 / 328411a0. S2CID 4335797.
- ^ Reddi, Jeevananda (2008 yil 4-noyabr). Iqlim o'zgarishi haqidagi afsonalar va haqiqatlar. p. 32. Olingan 20 dekabr 2018.
- ^ "Ozon teshigi yopilmoqda, tadqiqot natijalari". ABC News. Avstraliya radioeshittirish komissiyasi. 2007 yil 16-noyabr.
- ^ "Yangi hisobotda ozon qatlami va iqlim o'zgarishi o'rtasidagi ikki tomonlama bog'liqlik yoritilgan". UNEP yangiliklar markazi. 2010 yil 16-noyabr.
- ^ "NOAA, NASA: Antarktika ozon teshigi 20 yil ichida eng kichik ikkinchi". 2012 yil 24 oktyabr.
- ^ Kuttippurat, Jayanarayanan; Nair, Prijitha J. (2017-04-03). "Antarktika ozon teshiklarini tiklash belgilari". Ilmiy ma'ruzalar. 7 (1): 585. Bibcode:2017 yil NatSR ... 7..585K. doi:10.1038 / s41598-017-00722-7. ISSN 2045-2322. PMC 5429648. PMID 28373709.
- ^ Kuttippurat, J .; Kumar, P .; Nair, P. J.; Pandey, P.C. (2018-11-21). "Ozon tiklanishining paydo bo'lishi Antarktida ozon yo'qotish to'yinganligining pasayishi bilan tasdiqlanadi". NPJ iqlim va atmosfera fanlari. 1 (1): 1–8. doi:10.1038 / s41612-018-0052-6. ISSN 2397-3722.
- ^ "Ozon o'zgarishiga bog'liq bo'lgan ozon teshigini o'rganish". Yer instituti - Kolumbiya universiteti. 2011 yil 22 aprel. Olingan 21 dekabr, 2012.
- ^ Shermeier, Quirin (2005). "Quyosh shamoli ozon qatlamini uradi". Tabiat. doi:10.1038 / yangiliklar050228-12. Olingan 28 may, 2016.
- ^ Dell'Amore, Kristin (2011 yil 22 mart). "Birinchi shimoliy qutb ozon teshigi hosil bo'ladimi?". National Geographic. Olingan 6 aprel, 2011.
- ^ a b Nemis tadqiqot markazlarining Helmholts assotsiatsiyasi (2011 yil 14 mart). "Arktika rekord darajada ozon yo'qotish arafasida". Science Daily. Olingan 6 aprel, 2011.
- ^ "Arktikadagi ozonli elak: ko'proq g'alati?". Scienceblogs.com. 2011 yil 25 mart. 2011 yil 4 aprelda asl nusxasidan arxivlangan. Olingan 6 aprel, 2011.CS1 maint: yaroqsiz url (havola)
- ^ a b v "Ozon teshigining rivojlanishi Evropaga yaqinlashmoqda". EurActiv. Arxivlandi asl nusxasi 2011 yil 4 aprelda. Olingan 6 aprel, 2011.
- ^ a b v "Arktikada ozon yo'qotilishi rekord darajada". BBC News Online. 2011 yil 2 oktyabr. Arxivlandi asl nusxasidan 2011 yil 2 oktyabrda. Olingan 3 oktyabr, 2011.
- ^ a b "2011 yilda misli ko'rilmagan Arktikada ozon yo'qotilishi, deydi NASA rahbarligi ostida olib borilgan tadqiqotlar" (Matbuot xabari). NASA. 2011 yil 2 oktyabr. Olingan 1 iyul, 2016.
- ^ Millan, Luis; Manni, Gloriya (2017-05-02). "Shimoliy yarim sharda qayta tahlil qilishda ozon teshiklari vakolatxonasini baholash". Atmosfera kimyosi va fizikasi bo'yicha munozaralar. 17 (15): 9277. Bibcode:2017ACP .... 17.9277M. doi:10.5194 / acp-2017-341.
- ^ Strahan, S. E.; Duglass, A. R.; Newman, P. A. (2013). "2011 yil mart oyida kimyo va transportning past arktik ozonga qo'shgan hissasi Aura MLS kuzatuvlaridan olingan". Geofizik tadqiqotlar jurnali: Atmosferalar. 118 (3): 1563–1576. Bibcode:2013JGRD..118.1563S. doi:10.1002 / jgrd.50181. hdl:2060/20120011691. ISSN 2169-8996. S2CID 128447261.
- ^ Zell, Xolli (2013-06-07). "NASA 2011 yilgi Arktika ozon teshigining aniq sabablarini". NASA. Olingan 2019-10-03.
- ^ Yer, Stefani Pappas 2013-03-11T23: 38: 39Z sayyorasi. "Arktika ozonining g'alati sababi" teshik "topildi". livescience.com. Olingan 2019-10-03.
- ^ "Arktikada noyob ozon teshigi ochiladi va bu katta". Tabiat. 27 mart 2020 yil.
- ^ Xarvi, Fiona (2020-04-07). "Arktika ustidagi ozon qatlamida rekord o'lchamdagi teshik ochildi". The Guardian. ISSN 0261-3077. Olingan 2020-04-08.
- ^ Lyuben, Aleks (2020 yil 8 aprel). "Endi ozon qatlamida yana bir teshik bor. Ajoyib".. Vitse-muovin.
- ^ "Yer yangiliklari: Xitoy olimlari Tibet ustida yangi ozon teshigini topdilar". Elainemeinelsupkis.typepad.com. 2006 yil 4-may. Olingan 6 aprel, 2011.
- ^ Shermeier, Quirin (1999 yil 22-fevral). "Buyuk Beyond: Arktikadagi ozon teshigi tashvishga solmoqda". Blogs.nature.com. Olingan 6 aprel, 2011.
- ^ Oskin, Beki (2012 yil 26-iyul). "Bo'ron bulutlari ozonda teshik ochishi mumkin". LiveScience. Olingan 13 mart, 2015.
- ^ Favvora, Genri (2012 yil 27-iyul). "Bo'ronlar AQShda ozon qatlamiga tahdid solmoqda, deydi". The New York Times. p. A1. Olingan 13 mart, 2015.
- ^ a b Xegerl, Gabriele S.; va boshq. "Iqlim o'zgarishini tushunish va unga qo'shilish" (PDF). Iqlim o'zgarishi 2007 yil: Fizika fanining asoslari. I ishchi guruhning iqlim o'zgarishi bo'yicha hukumatlararo hay'atning to'rtinchi baholash hisobotiga qo'shgan hissasi. Iqlim o'zgarishi bo'yicha hukumatlararo hay'at. p. 675. Olingan 1 fevral, 2008.
- ^ "Ozonning yemirilishi". UNEP / DEWA / Earthwatch. 16 yanvar 2010. Arxivlangan asl nusxasi 2010 yil 16 yanvarda.
- ^ "Stratosferadagi iqlim o'zgarishida ozon va boshqa issiqxona gazlarining nisbiy roli". Suyuqlik geofizikasi laboratoriyasi. 2004 yil 29 fevral. Asl nusxasidan arxivlangan 2009 yil 20 yanvar. Olingan 13 mart, 2015.CS1 maint: yaroqsiz url (havola)
- ^ Silverman, Emi (1995 yil 4-may). "Freon Easy". Feniks yangiliklari. Olingan 6 aprel, 2011.
- ^ Savollar, I qism, 1.3-bo'lim.
- ^ Fabian, P .; Borchers, R .; Krüger, B. C .; Lal, S. (1985). "CFC-114 (CClF2-CClF2) ning atmosferada vertikal tarqalishi". Geofizik tadqiqotlar jurnali. 90 (D7): 13091. Bibcode:1985JGR .... 9013091F. doi:10.1029 / JD090iD07p13091.
- ^ ozon qatlamini yo'qotish bo'yicha savollar, II qism, 4.3-bo'lim
- ^ Yokouchi, Y .; Noijiri, Y .; Barri, L. A .; Tom-Sauntri, D. Machida, T .; Inuzuka, Y .; Akimoto, X .; Li, H. -J .; Fujinuma, Y .; Aoki, S. (2000). "Tropik qirg'oq quruqligidan atmosferaga kuchli metilxlorid manbai". Tabiat. 403 (6767): 295–298. Bibcode:2000. Nat.403..295Y. doi:10.1038/35002049. PMID 10659845. S2CID 4318352.
- ^ a b ozon qatlamini yo'qotish bo'yicha savollar, II qism, 4.4-bo'lim
- ^ Zuev, V.V .; Zueva, N.E .; Savelieva, E.S .; Gerasimov, V.V. (2015). "Erebus vulqonidan chiqadigan chiqindilar natijasida Antarktika ozon qatlamining pasayishi". Atmosfera muhiti. 122: 393–399. Bibcode:2015 yil AtmEn.122..393Z. doi:10.1016 / j.atmosenv.2015.10.005.
- ^ Dobson, G.M.B. (1968) Atmosferani o'rganish, 2-nashr, Oksford.
- ^ ozon qatlamini yo'qotish bo'yicha savollar, III qism, bo'lim 6. faqs.org
- ^ "ozon qatlamini yo'qotish bo'yicha tez-tez so'raladigan savollar, Antarktika". Faqs.org. Olingan 6 aprel, 2011.
- ^ Sheng Bo Chen, Liang Chjao va Yu LongTao (2017), "Tibet platosida stratosfera ozonining o'zgarishi", Atmosfera ifloslanishini o'rganish, 8 (3): 528–534, doi:10.1016 / j.apr.2016.11.007CS1 maint: bir nechta ism: mualliflar ro'yxati (havola)
- ^ "Xalqaro Ozon qatlamini saqlash kuni, 16 sentyabr". www.un.org. Olingan 2020-04-22.
- ^ Kanada, atrof-muhit va iqlim o'zgarishi (2015-02-20). "Ozon qatlamining yemirilishi: Monreal protokoli". aem. Olingan 2020-04-22.
- ^ Andersen, Stiven O.; Sarma, K. Madhava (2002). Ozon qatlamini himoya qilish: Birlashgan Millatlar Tashkiloti tarixi. Tuproq. p. 272. ISBN 9781849772266.
Qo'shimcha o'qish
- Andersen, S. O. va K. M. Sarma. (2002). Ozon qatlamini himoya qilish: Birlashgan Millatlar Tashkiloti tarixi, Earthscan Press. London.
- Benedik, Richard Elliot; Butunjahon yovvoyi tabiat fondi (AQSh); Diplomatiyani o'rganish instituti. Jorjtaun universiteti. (1998). Ozon diplomatiyasi: sayyorani himoya qilishning yangi yo'nalishlari (2-nashr). Garvard universiteti matbuoti. ISBN 978-0-674-65003-9. Olingan 28 may, 2016. (Elchi Benedik Monreal protokoli bilan yakunlangan uchrashuvlarda AQShning bosh muzokarachisi edi).
- Chasek, Pamela S., Devid L. Dovni va Janet Uels Braun (2013). Global ekologik siyosat, 6-nashr, Boulder: Westview Press.
- Garo, Brayan (2013). Ehtiyotkorlikdan foydaga: Monreal protokolida atrof-muhitni muhofaza qilishning zamonaviy muammolari. Yel universiteti matbuoti. ISBN 978-0-300-17526-4. Arxivlandi asl nusxasi 2013-03-30 kunlari.
- Grundmann, Reyner (2001). Transmilliy ekologik siyosat: Ozonni qayta qurish. Psixologiya matbuoti. ISBN 978-0-415-22423-9. Olingan 28 may, 2016.
- Haas, P. (1992). Xloroflorokarbonlarni taqiqlash: Epistemik hamjamiyatning stratosfera ozonini himoya qilish bo'yicha harakatlari. Xalqaro tashkilot, 46 (1), 187-224.
- Parson, Edvard (2004). Ozon qatlamini muhofaza qilish: fan va strategiya. Oksford: Oksford universiteti matbuoti.
Tashqi havolalar
- Ozon qatlami da Curlie
- NOAA / ESRL ozon qatlami
- NOAA Ozonni yo'q qiladigan gaz indekslari
- Ozon teshigi
- MACC stratosfera ozon xizmati ozon qatlamining o'tmishi va hozirgi holati to'g'risida xaritalar, ma'lumotlar to'plamlari va tasdiqlash hisobotlarini etkazib beradi.
- Muqobil tabiiy sovutgichlarni sovutish texnologiyalari bo'yicha yashil sovutish tashabbusi
- "Ozon teshigi: Sayyorani qanday qutqardik" premyerasi 2019 yil 10 aprel PBS
